引用本文: 四川大學華西醫院肝癌MDT團隊. 肝細胞肝癌全程多學科規范化管理:華西醫院多學科專家共識(第二版). 中國普外基礎與臨床雜志, 2020, 27(9): 1062-1077. doi: 10.7507/1007-9424.202006102 復制
1 前言
原發性肝癌是目前我國第 4 位常見惡性腫瘤及第 2 位腫瘤死亡病因,嚴重威脅著我國人民的健康和生命[1-2],其中 85%~90% 為肝細胞肝癌(hepatocellular carcinoma,HCC,以下簡稱肝癌)。我國肝癌長期生存率不高的主要原因,首先是肝癌高危人群篩查沒有普及,早期診斷率低,導致 70%~80% 的患者在診斷時已經是中晚期[3-4];其次是多個學科都在診治肝癌,肝癌專科化和規范診治還需加強;再次是,雖然手術切除,包括肝切除術和肝移植術,是肝癌患者獲得長期生存最重要的措施,但肝癌切除術后 5 年復發轉移率高達 40%~70%[4-5]。四川大學華西醫院肝臟外科及相關學科一直力求規范診治肝癌患者,并建立和加強肝癌多學科綜合治療協作組(multidisciplinary team,MDT)工作,最大限度地發揮各個學科的專業優勢,使患者獲益最大化。為了及時反映近 3 年肝癌研究的最新成果,四川大學華西醫院肝癌 MDT 團隊,對 2017 年版《肝細胞癌切除術后復發轉移的防治:華西醫院多學科專家共識》 [5-6]進行了修訂,以規范和推進肝癌全程多學科規范化管理的新模式。
本共識中的證據等級分為 6 級,推薦意見為 5 級[7-8],見表 1 和表 2。
下載CSV
| 證據級別 | 描述 |
| Ⅰa | 證據源于對多項隨機對照研究的 Meta 分析結果 |
| Ⅰb | 證據源于至少 1 項設計良好的隨機對照研究結果 |
| Ⅱa | 證據源于至少 1 項設計良好的前瞻性非隨機對照研究結果 |
| Ⅱb | 證據源自至少 1 項設計良好的其他類型干預性臨床研究結果 |
| Ⅲ | 證據源于設計良好的非干預性研究,如描述性研究、相關性研究等 |
| Ⅳ | 證據源于專家委員會報告或權威專家的臨床經驗報道 |
下載CSV
| 證據等級 | 描述 |
| A | 良好的科學證據提示該醫療行為帶來明確獲益,建議醫師對患者實施該醫療行為 |
| B | 現有證據表明該醫療行為可帶來中度獲益,超過其潛在風險;醫師可建議對患者實施該醫療行為 |
| C | 現有證據表明該醫療行為可能獲益較小,或獲益與風險接近;醫師可根據患者個體情況有選擇地向患者建議或實施該醫療行為 |
| D | 現有證據表明該醫療行為無獲益,或其潛在風險超過獲益;醫師不宜向患者實施該醫療行為 |
| E | 缺乏科學證據,或現有證據無法評價該醫療行為的獲益與風險;醫師應幫助患者理解該醫療行為存在的不確定性 |
2 從肝臟占位性病變初診到診斷肝癌
2.1 肝癌的診斷標準
2.1.1 臨床診斷標準
國際上公認,可以采用臨床診斷標準的實體瘤,唯有肝癌[3-4, 9]。臨床診斷肝癌主要依據 3 個方面:即慢性肝病背景、影像學特征及血清 AFP 水平。因肝癌巨大的異質性,影像學表現復雜多樣、AFP 水平差異較大,實際應用時需綜合分析、嚴格掌握標準。目前國際上公認的臨床診斷標準為:同時滿足以下條件中的 2.1.1.1+2.1.1.2.1+2.1.1.3 三項或 2.1.1.1+2.1.1.2.2 兩項;否則,應進一步穿刺活檢[4, 6, 9-10]。
2.1.1.1 肝病史
具有肝硬變以及 HBV 和(或)HCV 感染 [HBV 和(或)HCV 抗原陽性] 的證據,或非酒精性脂肪肝病史[10]
2.1.1.2 典型的肝癌影像學特征
MRI 和(或)CT 動脈增強掃描或增強多期掃描顯示肝內病灶在動脈期不均勻或均勻強化,即富血供(arterial hypervascularity),而門靜脈期或延遲期快速洗脫(venous or delayed phase washout)。MRI 肝膽特異性對比劑釓塞酸二鈉增強掃描,動脈期均勻或不均勻強化、門靜脈期無快速洗脫,但肝膽期無強化;或動脈期無強化,門靜脈期無強化或輕度強化,肝膽期無強化,且病灶在高 b 值 DWI 圖像呈高信號[11-12]。美國放射學會開發的肝臟影像報告和數據系統(liver imaging reporting and data system,LI-RADS)對肝癌和復發性肝癌(recurrent HCC,RHCC)影像學的解讀有一定幫助[13]。
2.1.1.2.1
如果肝臟病灶直徑為 1~2 cm,需要 CT 和 MRI 兩項檢查都顯示具有肝癌典型影像學特征,可診斷肝癌,以加強診斷的特異性。
2.1.1.2.2
如果肝臟病灶直徑>2 cm,CT 和 MRI 兩項檢查中有一項顯示肝臟占位具有肝癌典型影像學特征,即可診斷肝癌。
2.1.1.3 血清 AFP
AFP水平≥400 μg/L 持續 1 個月或≥200 μg/L 持續 2 個月,并能排除其他原因引起的 AFP 升高,包括妊娠、生殖系胚胎源性腫瘤、活動性肝病、消化道腫瘤等。GALAD 和 BALAD 評分系統,主要利用肝癌相關標志物 AFP、AFP-L3、異常凝血酶原(des-γ-carboxy prothrombin,DCP)水平等構建的數學模型,可提高早期肝癌的檢出率,越來越受到歡迎[14-15]。
2.1.2 病理學診斷標準
肝臟占位病變或者肝外轉移灶的穿刺活檢組織或手術切除標本,經細胞學和(或)病理組織學檢查診斷為肝癌,此為診斷的金標準。肝癌的病理學診斷規范由標本處理、標本取材、病理學檢查、病理報告等部分組成,對手術切除標本,推薦采用“7 點”基線取材法和病理報告的結構化報告格式,盡可能反映出肝癌復發轉移的高危因素[16]。
2.1.3 RHCC 的診斷標準
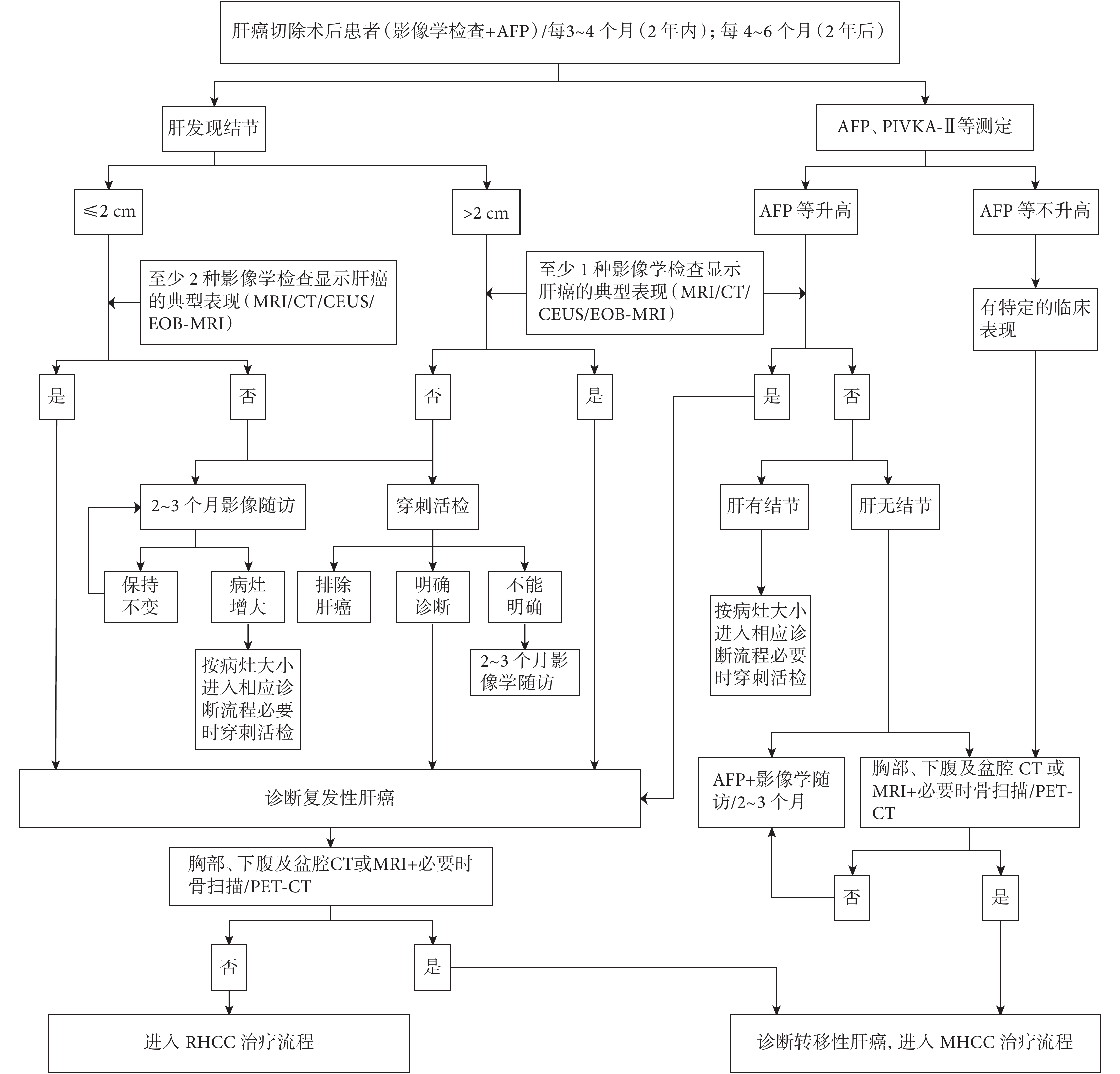
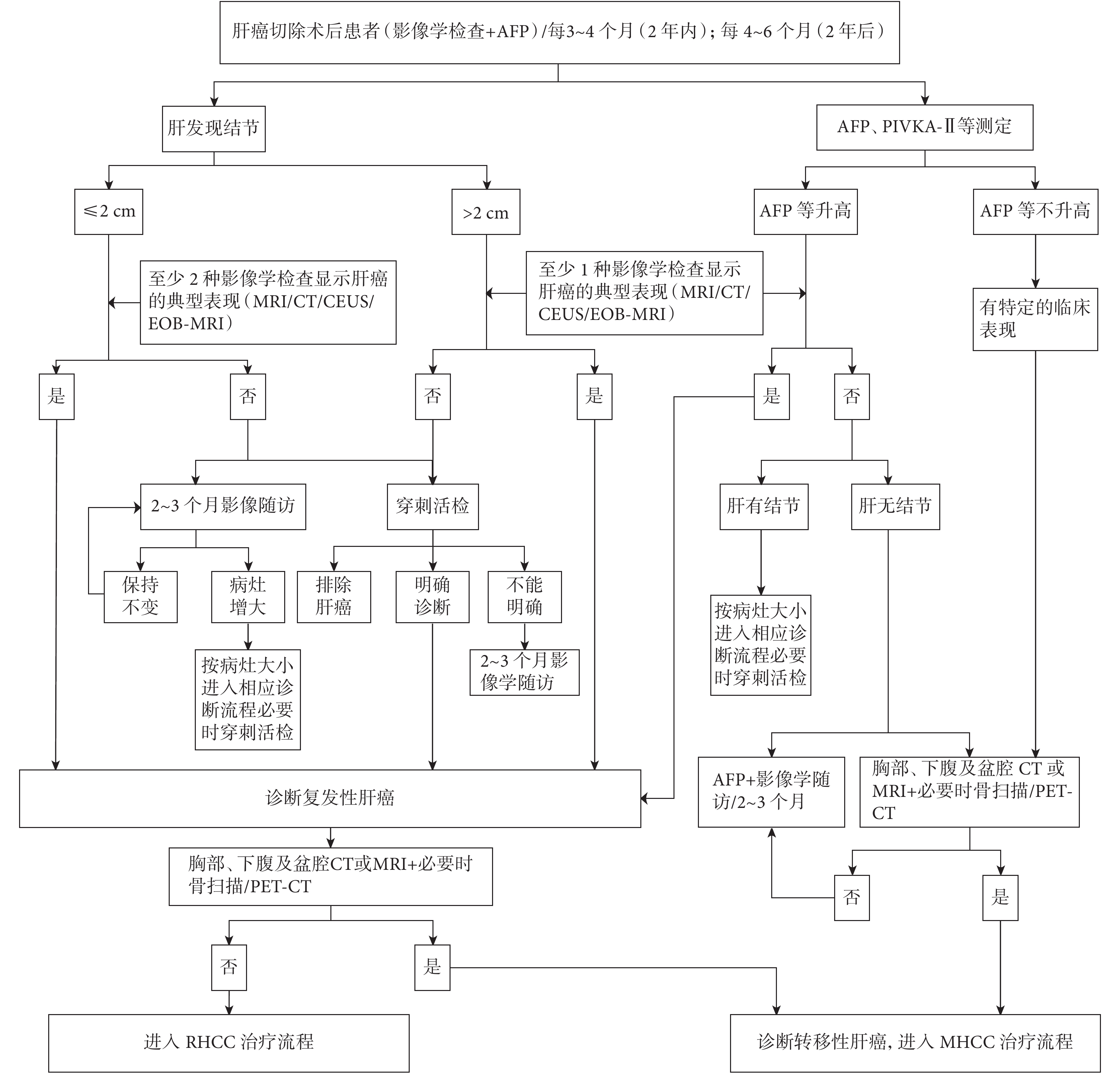
與初次肝癌診斷標準相同,甚至要求兩種影像學檢查顯示肝癌的血供特征[4, 17-20]。兩種甚至三種影像學檢查可優勢互補[4],對準確的 RHCC 預后預測和幫助選擇最佳治療方式有積極意義。在慢性肝病背景下,肝內實性病灶的定性,推薦采用 MRI 肝膽特異性對比劑增強掃描,對鑒別治療后壞死灶、出血灶、再生結節與 RHCC,是目前國際上公認的準確的影像學檢查方法。在 RHCC 診斷明確,進入治療之前,或未診斷 RHCC 而 AFP 升高、或伴有癌轉移其他臨床表現的患者,應進行胸部 CT 和全身骨顯像甚至 PET-CT 檢查,以診斷轉移性肝癌(metastatic HCC,MHCC) [4, 20]。復發性轉移性肝癌診斷路徑見圖 1。
2.1.4 肝占位病變初診患者的檢查評估目標
肝癌患者通常在多年慢性乙肝感染或(和)慢性丙肝感染的基礎上發生肝硬變,進而可發生肝癌[4],有的患者其肝臟還伴有肝囊腫和(或)血管瘤等,那么通過病史、乙肝及丙肝標志物檢查、腫瘤標志物檢查、影像學檢查等綜合判斷的目標,就是明確患者肝臟占位性病變中有沒有肝癌及有幾個肝癌病灶。
2.2 不能切除肝癌的轉化切除
對初診不能夠切除的肝癌進行介入治療、靶向治療、化療、放射治療和免疫治療,其中達到部分緩解和完全緩解的患者,再次評估后可進行第二步切除,也稱降期切除,現統稱為轉化切除[21]。轉化切除是提高肝癌切除率的重要途徑,是不能夠切除肝癌經過綜合治療后達到的最好目標。轉化治療的途徑包括以增加剩余肝體積為主要目標的肝癌轉化切除,包括門靜脈栓塞(portal vein embolization,PVE) [22]、聯合肝臟離斷和門靜脈結扎的二步肝切除術(associating liver partition and portal vein ligation for staged hepatectomy,ALPPS) [23-25]、肝靜脈系統栓堵術(liver venous deprivation,LVD) [26-27]等措施;以縮小肝癌為主要目標的轉化切除,包括肝動脈化療栓塞(transcatheter arterial chemoembolization,TACE) [28]、系統治療(靶向治療、免疫治療和化療) [29-30]、放療等方式;以促進剩余肝增生和縮小肝癌兩個維度[31]都實施的轉化治療等多種方法,值得深入研究。
2.3 可切除肝癌術前對預后的預測和考慮
2.3.1 肝癌肝切除術前對復發高危風險的預測
肝癌肝切除術后復發高危風險是指伴血管癌栓、微血管侵犯(microvascular invasion,MVI)和多結節肝癌,中危風險是指肝癌直徑大于 5 cm[32]。大體病理觀察和影像學檢查顯示,單結節肝癌的預后好于多結節融合型肝癌[33-35]。隨著肝癌的增大,發生 MVI 的可能性逐漸增加[36]。有研究[37]顯示,肝癌病灶大小(直徑截點值 3.6 cm)、DCP(截點值 101 mAU/mL)和最大標準化攝取值(the maximum standardized uptake value,SUVmax,截點值 4.2)構建的評分系統,預測 MVI 的敏感度與特異度可達 100% 和 90.9%。Lee 等[38]用釓塞酸二鈉增強 MRI 術前預測單個肝癌伴 MVI 的研究顯示:動脈期的腫瘤邊周強化、腫瘤邊緣不光滑和肝膽期腫瘤周邊低增強等三項或其中的兩項,可作為術前預測 MVI 的影像學特征,其特異度超過 90%。沈鋒團隊[39]用 AFP 水平、HBV-DNA 水平、血小板計數、腫瘤影像學特征、影像學腫瘤個數、影像學腫瘤大小和影像學腫瘤包膜 7 項指標創作列線圖,用于術前預測乙肝相關性 Milan 標準肝癌伴發 MVI 的研究顯示:積分大于 200 屬于伴 MVI 高風險組,其陽性預測值達 57.4%。術前影像學檢查顯示 4 個及以上的多結節肝癌不首選手術切除[4]。Li 等[40]發現,多結節肝癌無論符合 Milan 標準與否,肝移植的預后優于肝切除。Li 等[41]發現門靜脈主干癌栓或 3 個結節肝癌伴 AFP 高于 400 μg/L 者行肝切除治療為無益肝切除。門靜脈癌栓和(或)淋巴結轉移患者不適合接受肝移植[42-43]。肝癌干細胞標志物高表達[44]和表達上皮細胞黏附分子(epithelial cell adhesion molecule,EpCAM)的循環腫瘤細胞(circulating tumor cells,CTC,EpCAM+CTC7.5)≥2[45]提示預后不良。
若預測到某患者存在 MVI,需怎么做呢?目前我們可能應改進原來的處理思路,不宜對該患者推薦射頻治療,該患者即便行肝移植也易復發,可考慮行解剖性肝切除和(或)寬切緣切除術及術后輔助治療,也可考慮行新輔助治療以改善預后[39, 46]。
2.3.2 肝癌的新輔助治療
因為介入技術與放療技術的進步和藥物的涌現,在肝癌可切除但預后不佳的患者中,已初步顯現術前新輔助治療可以改善預后,讓患者更多獲益。Allard 等[47]報道,對 TACE 有病理學反應的肝癌患者在肝切除或肝移植后的預后好于無或較差病理學反應的肝癌患者。程樹群團隊[48]報道的一項多中心 RCT 顯示:術前加用三維適形放療可明顯改善肝癌伴 PVTT 患者行肝切除術后的無復發生存率(RFS)和總體生存率(OS)。
3 肝癌切除術中預防復發轉移的措施
右肝大肝癌,特別是影像學檢查提示膈肌受侵者,應行前入路肝切除[49-50]。如預估斷肝出血達到 600~800 mL,應行入肝血流阻斷或半入肝血流阻斷[51-52]。術中大量出血、輸血會影響患者的預后[53];而入肝血流阻斷不會影響腫瘤患者的預后[54]。肝癌手術中超聲甚至超聲造影[55]可進一步顯示癌旁有無衛星結節、癌栓、余肝有無結節、病灶與第一、第二和第三肝門的關系,并幫助確定切線。ICG 熒光技術也可發現衛星結節、幫助確定解剖性肝切除的切線等[56]。如術中發現明確或可疑病灶,應考慮同時切除或消融治療[57-58]。根據腫瘤范圍、肝硬變程度、肝功能與肝儲備功能、預測的殘肝體積等應依次選擇解剖性肝切除、或寬切緣非規則性肝切除、或腫瘤局部切除術,以盡可能切除癌周可能存在的 MVI 和衛星結節[46, 59-60]。
4 肝癌切除術后預防復發轉移的措施和定期隨訪
4.1 肝癌根治性切除標準[3 -5 , 18 ]
4.1.1 術中判斷標準
① 肝靜脈、門靜脈、膽管以及下腔靜脈未見肉眼癌栓。② 無鄰近臟器侵犯,無肝門淋巴結或遠處轉移。③ 腫瘤切緣>1 cm;若切緣<1 cm,但切除肝斷面組織學檢查無腫瘤細胞殘留,即切緣陰性。④ 術中超聲和 ICG 熒光技術未發現有衛星灶、小脈管癌栓、新病灶等。
4.1.2 術后病理報告判斷標準
規范性病理取材的病理報告[16]未報 MVI、衛星結節、切緣陽性等。
4.1.3 術后 2 個月判斷標準
① 術后 2 個月行超聲、CT、MRI(必須有其中兩項)檢查未發現腫瘤病灶;② 如術前 AFP 和(或)DCP 升高,則要求術后 2 個月行 AFP 和(或)DCP 定量測定,其水平在正常范圍(極個別患者 AFP 降至正常的時間超過 2 個月)。
4.2 肝癌切除術后伴有復發轉移高危因素患者的預測、監測與治療
4.2.1 肝癌切除術后復發轉移的高危因素
肝癌切除術后 1~2 個月,應結合術中情況、AFP 和 DCP 下降情況及病理報告,再次評估患者的復發轉移高危因素,制定應實施的輔助治療,甚至作出預后基本預測。若血小板淋巴細胞比(PLR)>107、MVI 陽性和單個肝癌病灶直徑≥6.8 cm 3 個因素同時存在,提示預后差[61]。導致肝癌術后復發轉移的危險因素很多:① 手術因素,包括非解剖型肝切除[62]、窄切緣[60]、手術切緣腫瘤殘留[63]、較大量出血輸血[64]、術中擠壓腫瘤[49]等;② 臨床病理因素,包括腫瘤低分化、腫瘤分期較晚[4]、腫瘤破裂[65]、無完整包膜、腫瘤直徑大>5 cm、腫瘤數目≥3 個[66-67]、脈管侵犯[46]、淋巴結轉移[68]、衛星灶、鄰近器官受侵[4]、AFP 明顯升高[67]、AFP 術后 2 個月未降至正常水平[69]、術后血管造影見殘存陽性病灶等;③ 背景肝病因素,包括肝炎病毒感染[70]、肝硬變等。
4.2.2 肝癌切除術后伴有復發轉移高危因素患者的治療
對于肝癌切除術后伴有復發轉移高危因素的患者,目前認為應考慮輔助治療[4-6, 71],以預防腫瘤復發。已有較多研究[32, 71-77]顯示,對伴有明確復發轉移風險如 MVI、多結節和腫瘤直徑>5 cm 的肝癌切除術后患者,術后輔助 TACE 治療,可使患者生存獲益。對伴有復發轉移高危因素患者根據情況還可進行聯合治療,包括胸腺肽 α1[78-80]或 α-干擾素[81-85]、索拉非尼[86-88]或侖伐替尼[89-90]、維生素 K2[91-94],還可考慮聯合化療[95-96]等。
4.2.3 肝癌切除術后患者的定期隨訪
目前證據表明,對于沒有復發轉移中高危因素的患者采取不恰當的輔助治療如 TACE,可能引起殘余肝臟的損害,導致肝功能惡化而致生活質量下降,反而影響長期生存,甚至可使肝外轉移的發生率升高,預后更差[72, 77]。乙肝或丙肝相關性肝癌,根據情況需要行抗病毒治療[70, 97-99]。
肝癌患者術后應進行定期隨訪[4-6, 71, 100],內容主要包括[4, 6, 71]肝臟影像學檢查、肝癌標志物(AFP 和 DCP)檢查以及肝功能檢查,隨訪頻度在 2 年內是每 3~4 個月隨訪 1 次,以后則可適當延長。HBV-DNA 的復查根據肝功能情況、是否抗病毒治療等進行選擇。
5 肝癌切除術后復發和轉移
肝癌病情的進展多見于肝內,如肝內病灶長大、門靜脈侵犯、術后復發等,而遠處轉移相對較少,約 13.5%~42%[101-103]。
5.1 肝癌切除術后復發模式及臨床意義
目前廣泛認可的 RHCC 來源于:① 肝癌切除后,肉眼難以查見的殘留腫瘤細胞繼續生長或通過肝內血運播散形成的肝內轉移(intrahepatic metastasis,IM);② 由于 HBV 或 HCV 感染,肝臟在長期慢性炎癥反應及肝硬變背景下,正常肝細胞染色體長期累積突變而發生的惡性轉化,形成多中心發生(multicentric occurrence,MO)的 RHCC。
最早的鑒別方法是基于臨床病理資料的總結,根據 RHCC 復發時間,將 1 年內復發的 RHCC 定為 IM,是為早期復發,1 年以上復發的 RHCC 定為 MO,是為晚期復發[104-105]。MO 的總體生存率好于 IM。也有基于病理診斷判定 IM 的標準[106]以及基于腫瘤的分化情況提出 MO 的鑒別方法[107-108],但其敏感度和特異度均不理想,很大程度受到病理醫生主觀因素的影響。隨著分子生物學技術及基因組學技術的發展,臨床及病理學者對 RHCC 來源探索了比較多的鑒別方法,包括微衛星雜合性缺失模式(loss of heterozygosity,LOH)、微衛星不穩定性檢測(microsatellite instability)、p53 基因點突變模式分析(TP53 gene mutation analysis)、X 染色體失活性分析(X chromosome inactivation analysis)、HBV-DNA 整合位點檢測(HBV-DNA integration detection)、DNA 甲基化模式檢測(DNA methylation analysis)、miRNA 譜試分析、比較基因組雜交分析(comparative genomic hybridization,CGH)等[109-118]。有學者[119-120]綜合多組學的方法并聯合臨床病理資料,鑒定多結節 HCC 的發生來源及腫瘤異質性。其中 LOH 運用廣泛。微衛星 DNA 是反應細胞 DNA 整體穩定性的良好標志物,聯合多個高頻 LOH 染色體能提高鑒別 RHCC 的準確性。所需標本條件較易達到,甲醛固定石蠟包埋的樣本或穿刺標本都可滿足檢測要求[109]。
肝癌的復發轉移時間極其混亂[104-105, 121-123],從復發肝癌患者手術切除的標本進行克隆分析顯示[112],“復發”腫瘤有來自原發癌的,也有為新生腫瘤的。臨床上,以復發時間 1 年為界判斷為早期復發還是晚期復發,符合絕大多數患者的肝癌復發情況[6, 104-105],簡單實用。原則上,對于 1 年內復發的肝癌即 IM,有時貌似可以切除,但應選擇消融治療、放療、TACE 聯合靶向治療等微創治療手段;而對于 1 年以上復發的肝癌即 MO,可選擇再切除或肝移植等根治性治療措施[6,112]。
5.2 復發性肝癌的治療
5.2.1 復發性肝癌治療路線圖
目前已經明確,初治肝癌為多個腫瘤、伴 MVI 等,是肝癌切除術后的復發高危因素;同時,初次肝癌手術切除 1 年以內還是 1 年以上復發,治療方式選擇的原則不同。我們基于這個原則,并參考原發性肝癌診療規范(2017 年版) [3]建立了一個初步的 RHCC 治療流程[6],見圖 2。
5.2.2 復發性肝癌再切除
5.2.2.1 復發性肝癌再切除
有研究[124-125]顯示,肝切除術后的可切除性復發肝癌,再次肝切除者的總生存率(OS)優于 TACE 治療者;系統評價也顯示出了相同的結論[126];甚至有研究[127-128]認為,患者能夠從第 3 次手術切除中生存獲益;而第 4 次肝切除并不會改善患者的生存情況[129]。對于可切除的肝外轉移灶,手術切除同樣能夠給患者帶來生存獲益[130]。復發肝癌患者再次手術的預后與患者初次手術時的臨床病理特征、復發間隔時間等密切相關[131]。再次切除患者最好是初次手術時腫瘤單發、腫瘤復發時間間隔≥1 年,而且復發時腫瘤無血管侵犯[6, 132]。復發肝癌患者再次的術前評估與初次術前評估一樣,應該考慮患者的體能狀態、肝功能、肝儲備功能、肝硬變程度、門靜脈高壓程度、剩余肝臟體積等[127, 133-135]。再次肝切除要求患者的 ECOG PS 評分為 0~1 分,應無明顯的心、肺、腦、腎等重要器官功能障礙;肝功能 Child-Pugh A 級或者肝功能 B 級經短期護肝治療后恢復到 A 級;肝臟儲備功能良好。患者的剩余肝臟體積(FLV)應結合患者的肝功能、肝臟儲備功能等多項指標綜合考慮[135]。對肝硬變、Child-Pugh A 級、ICG R15<10% 的患者,FLV 應>40%;而 ICG R15 為 10%~20% 的患者,FLV 應>50%[136-138];對于肝纖維化的患者,FLV 應>30%[139];而對于正常肝臟患者,FLV 應>20%[139]。高齡患者經過嚴格評估后,行再次肝切除同樣是安全可行的[140]。
5.2.2.2 可切除性復發肝癌的腹腔鏡手術
與傳統手術相比,腹腔鏡肝切除手術具有創傷小、術后恢復快等優點。許多研究亦證實,對于復發肝癌患者,腹腔鏡手術后無瘤生存率及總體生存率與傳統開腹手術無明顯差異[141-145]。但值得注意的是,這些研究中的許多腹腔鏡手術,對患者的腫瘤大小和位置等已有選擇[142-143, 146]。近年一項多中心研究證實,對于腹腔鏡肝切除術中中轉開腹的患者術中出血量、輸血量、術后住院時間、并發癥發生率和死亡率均明顯增加[147]。
5.2.2.3 可切除性復發性肝癌伴門靜脈癌栓的手術適應證
肝癌合并門靜脈癌栓是否應接受手術切除一直存在較大爭議,近年來有較多文獻報道此類患者中的部分患者可從手術中獲益。亞太地區學者認為對于肝癌合并門靜脈癌栓的部分患者應考慮手術切除,但應重視患者篩選[42, 148-149]。對于復發肝癌伴門靜脈癌栓,更應嚴格把握此類患者的手術切除適應證。首次根治性切除術后復發時間大于 1 年,為可切除性復發腫瘤,門靜脈癌栓程氏分型屬于Ⅰ、Ⅱ型,適合肝段、肝葉等解剖性肝切除,可考慮行肝葉、半肝等解剖性肝切除并整塊切除癌栓[42]。但肝癌合并Ⅲ型門靜脈癌栓患者預后差,目前基本不首選手術切除[40, 42]。
5.2.2.4 可切除性復發性肝癌伴膽管癌栓的手術適應證
初次手術切除肝癌不伴 MVI 等復發高危因素、復發時間 1 年以上的肝癌肝內復發伴膽管癌栓,原則上應爭取外科手術完整切除腫瘤及取盡癌栓,特別是對那些不伴梗阻性黃疸的患者[150-154]。腫瘤個數小于 3 個、局限在肝段或肝葉、預估有足夠的殘余肝體積、癌栓位于腫瘤側膽管,可行腫瘤及癌栓累及膽管的解剖性肝切除。復發肝癌可切除,癌栓進入膽總管,癌栓形成時間較短尚未發生機化,可行肝葉、半肝等的解剖性肝切除加膽管癌栓取出術加 T 管引流術[152-153, 155];如癌栓形成時間長、癌栓可能侵及膽管壁但局限在肝外膽管、復發肝癌可切除,可考慮行解剖性肝切除及肝尾葉切除加肝外膽管切除及膽腸吻合術[17, 150, 156-157]。
5.2.2.5 可切除性復發性肝癌伴肝硬變、門靜脈高壓癥、脾大及脾功能亢進聯合脾切除術適應證
復發性肝癌具有可切除性,伴中、重度脾臟腫大,重度脾功能亢進(WBC <3×109/L,PLT <50×109/L)表現者,應該考慮行肝癌切除聯合脾切除[158-163]。有明顯食管胃底靜脈曲張,特別是發生過食管胃底曲張靜脈破裂大出血患者,經過嚴格的評估后還應同時行賁門周圍血管離斷術。對有嚴重胃黏膜病變者,如患者術中情況允許,應行脾腎分流術或者其他類型的選擇性門腔分流術。此類患者都應考慮到將來有行肝移植的可能性,因此不宜行影響第一肝門的分流術。此類聯合手術宜從嚴掌握,如果患者既往有肝功能不全、凝血功能障礙等病史,應考慮行挽救性肝移植。
5.2.3 復發性肝癌消融治療
局部消融治療的消融方式包括:射頻消融(RFA)、微波消融(MWA)、高功率超聲聚焦消融(HIFU)、冷凍治療(CRA)、無水乙醇注射治療(PEI)等[164-166]。
較多研究[167-171]顯示,肝癌切除術后復發肝癌患者可以從 RFA 中獲益,甚至可取得與再切除相近的 OS 及 DFS。與切除相比,射頻消融的明顯優勢在于微創、恢復快、并發癥少、可重復實施性強,還可忽略首次手術時是否存在復發高危因素或復發時間。對于復發肝癌,單個腫瘤直徑≤5 cm;腫瘤數目≤3 個、其最大直徑≤3 cm;不伴有脈管內癌栓或鄰近器官受侵;超聲顯示有射頻路徑,腫瘤可達到完全消融,是射頻消融治療的指征[172-175]。對直徑<2 cm 的肝癌,RFA 作為一線治療[10]。需要特別注意的是,對于直徑>3 cm 的腫瘤,射頻消融中應采用多點覆蓋,以確保病灶完全消融[176],或選擇 MWA 消融[177-182]。
有較多研究[183-184]比較了 CRA、RFA 和 MWA 治療直徑<5 cm 肝癌的療效,結果發現,上述 3 種治療的 OS 和 DFS 無差異;但對于直徑為 3~4 cm 的腫瘤,CRA 術后局部復發率較 RFA 更低。有研究提示 HIFU 和 RFA 對于符合米蘭標準的復發性肝癌治療效果相當[185];而 PEI 對直徑≤2 cm 的肝癌消融效果確切[186-189]。
5.2.4 復發性肝癌行肝移植
許多研究[190-193]報道,對于復發性肝癌行挽救性肝移植可以生存獲益。甚至有研究[191-192]認為,復發性肝癌行挽救性肝移植,OS 明顯優于行再次手術切除。在不少研究中,初次手術時的腫瘤符合米蘭標準[191, 193-195],也有初次手術時腫瘤超過米蘭標準[196-197],但多數研究中,患者初次手術時均無大血管癌栓。在行挽救性肝移植時,大多數中心要求腫瘤情況符合米蘭標準[190-191, 194-195, 198],但有些中心采用擴展標準,如京都大學標準[199]、九州大學標準[192]、杭州標準[196]、up-to-7 標準[197]。許多研究[190, 192, 199]亦證實,對于復發肝癌患者,活體肝移植是安全有效的。但目前尚缺乏針對復發肝癌患者尸體肝移植和活體肝移植預后比較的報道。
有研究[200-202]顯示,肝癌切除術后發現有復發高危因素時就登記肝移植,即預防性肝移植,可能比挽救性肝移植效果更好。但需更多的研究數據證實。
5.2.5 復發性肝癌的 TACE 治療
早期的 RCT 研究[203-204]顯示,局限在肝內且沒有血管侵襲的中晚期肝癌患者可從 TACE 治療中獲益。TACE 序貫治療,其中約 10% 的肝癌患者可以達到完全緩解[205]。肝癌負荷大小是決定 TACE 治療效果的主要因素,肝癌負荷小的,TACE 效果好[206]。對于復發性肝癌數目≤3 個,直徑≤5 cm 時,TACE 和 RFA 的效果相當[207],尤其是對于切除或移植術后多發肝內復發的患者,TACE 是降低復發后死亡率的重要手段[208]。還有研究[209]顯示,符合米蘭標準的復發肝癌,早期復發時 TACE 和 RFA/切除具有相同效果,晚期復發時 RFA/切除效果好于 TACE。Jin 等[209] 比較了 TACE、切除和 RFA 對伴 MVI 的復發肝癌的治療效果,結果顯示 TACE 治療 MVI 陽性復發肝癌可獲得較切除和 RFA 更好的 OS 和 DFS,特別對于 1 年內復發的 MVI 陽性者,OS 顯著高于切除和 RFA 組。Yang 等[210]回顧性研究了 TACE 聯合 RFA 與單獨行 TACE 或 RFA 治療復發性肝癌的效果,聯合治療組的 5 年生存率顯著高于單獨 TACE 或 RFA 組。TACE 治療復發肝癌的指征[210-211]:① 復發性肝癌臨近重要血管或膽管而無法行切除或 RFA 術;② 肝內多發復發性腫瘤;③ 早期肝內復發性肝癌(1 年內);④ 患者個人愿意選擇 TACE 治療。
5.2.6 復發性肝癌的放射治療
放射治療(簡稱放療)分為外放療和內放療。外放療是利用放療設備產生的射線(光子或粒子)從體外對腫瘤照射。內放療是利用放射性核素,經機體管道或通過針道植入腫瘤內[4]。
5.2.6.1 肝癌外放療技術[4 ]
包括三維適形或調強放療、圖像引導放療(image guided radiation therapy,IGRT)或立體定向放療(stereotactic body radiation therapy,SBRT)等技術。外放療已經成為治療復發性肝癌有效和安全的手段之一[212-214],國內外研究報道小肝癌行放療 5 年生存率可達 60% 以上[213-215],甚至療效等同于 RFA 治療[216],但與手術相比尚無直接證據。復發性肝癌患者常面臨腫瘤位于切除困難位置、多發、伴大血管癌栓及肝功能失代償等,也常伴發肺、骨等部位的肝外轉移,可根據腫瘤的情況選擇合適的放療方案,可多次實施以縮小肝內外病灶,控制腫瘤進展,減輕腫瘤引起的癥狀,以延長患者的生存時間。如肺轉移患者經體外放射治療 2 年生存率可達 70.7%,骨轉移患者經體外放射治療中位生存期達到 7.4 個月[217-221]。近年來,對于肝癌局部明顯進展如伴門靜脈癌栓和肝靜脈/腔靜脈癌栓,加用外放療取得了較明顯療效[48, 222]。
5.2.6.2 內放療技術[4 , 223 ]
即放射性粒子植入技術,包括組織間植入、門靜脈植入、下腔靜脈植入和膽管內植入,分別治療肝內病灶、門靜脈癌栓、下腔靜脈癌栓和膽管內癌或癌栓。放射性粒子包括90Y 微球、131I 單克隆抗體、放射性碘化油、125I 粒子植入等。已有動脈灌注90Y 微球治療肝癌肝移植術后復發,可有效控制復發癌進展的報道[224]。氯化鍶(89Sr)發射出 β 射線,可用于靶向治療肝癌骨轉移病灶[225]。肝癌患者接受 TACE 放射微球[226]治療能獲得更高的腫瘤壞死率和更低的腫瘤進展率。但其在復發性肝癌中的作用尚待研究。
5.2.7 復發性肝癌的聯合或序貫治療
在中國,目前肝癌診治領域的特點仍然是多方法、多學科共存,而以治療手段建科的分科診療體制與實現有序規范的肝癌治療之間存在一定的矛盾[3],因此肝癌 MDT 下規范治療的選擇與綜合治療極其重要,特別是對復發性肝癌的治療。復發肝癌切除聯合術中射頻治療,已進行抗病毒治療的復發肝癌切除術后伴有復發轉移高危風險患者再序貫 TACE、靶向治療與胸腺肽 α1 治療,及肝內復發肝癌 RFA 序貫 TACE 治療[211, 227]等,都是聯合或序貫治療的基本模式,能夠降低腫瘤負荷,延長患者生存。
5.3 肝癌切除術后肝外轉移
肝癌病情進展多見于肝內,而遠處轉移相對較少,為 13.5%~42%[101-103]。肝癌患者死亡原因也更多為肝功能衰竭和肝內腫瘤引起的其他相關并發癥。肝癌常見的肝外轉移部位是肺,其次是淋巴結、骨、腎上腺、腹膜等,也可直接侵犯臨近器官[101, 103]。
肝癌切除術后肝外轉移的發生可概括為 3 種模式[103]:① 先肝內復發后肝外轉移;② 同時性肝內復發肝外轉移;③ 首先發生肝外轉移。Yang 等[103]對 348 例術后患者隨訪(4.8±3.7)年,發現肝內復發、肝外轉移分別為 64% 和 14%。3 種肝外轉移模式中,患者發生肝外轉移后總生存時間并無明顯差異,但先肝內復發后肝外轉移模式中的肝外轉移出現時間最晚,分別為(3.2±0.8)年、(0.8±0.5)年和(0.9±0.2)年。
盡管肝癌切除術后復發轉移以肝內復發多見,但術后隨訪復查不能忽視肝外轉移的可能性。結合 AFP、DCP、彩超、CT、MRI 等常規檢查手段,早期發現肝內復發和(或)肝外轉移,避免漏診,才能為后續治療提供準確依據。18F-FDG PET 或 PET/CT 對于腫瘤診斷是很好的功能成像技術,然而對于肝癌特別是分化良好的肝癌,其敏感度相對較低。Meta 分析顯示 18F-FDG PET 或 PET/CT 對于肝癌復發轉移診斷的敏感度為僅為 64%,特異度為 95%[228-229]。因此,18F-FDG PET 或 PET/CT 并不作為肝癌診斷或術后隨訪的常規影像學檢查手段,但仍有助于發現隱匿的復發轉移病灶。
對于肝癌切除術后肝外轉移,主要考慮系統治療[89, 95, 230-231]。關于肝外轉移的局部治療,目前沒有高級別的循證醫學證據。但回顧性研究[232-234]顯示,手術、放療、消融等局部治療對部分腫瘤進展緩慢、轉移病灶少的高選擇性肝外轉移患者,可能帶來生存獲益,其回顧性的小樣本研究顯示:術前無病生存期>12 個月、肺轉移灶≤4 個、單純肺轉移、最大病灶直徑<3 cm 等可能與肺轉移術后預后相關。對于肝癌切除術后 1 年以上、單發淋巴結轉移或單純腎上腺轉移的患者,手術切除也可能獲得較長期生存[235-236]。骨轉移比肺轉移預后差,手術切除需謹慎,可考慮放療緩解癥狀,減緩病灶進展[225, 237-238]。
5.4 肝癌切除術后復發轉移的全身治療
5.4.1 抗病毒治療
TACE、手術和化療均可引起乙型肝炎病毒復燃,而且抗病毒治療可以降低肝癌切除術后復發率,改善患者的生存,因此,合并有乙肝病毒感染且復制活躍的肝癌患者,推薦口服核苷(酸)類似物抗病毒治療[70, 98-99]。
5.4.2 肝癌藥物治療
包括分子靶向治療、系統化療和免疫治療。
5.4.2.1 分子靶向治療
索拉非尼是一線治療晚期 HCC 的分子靶向藥物。有研究[86-88]顯示:索拉非尼作為輔助治療,對術后高危復發的患者也有一定的療效。一項大型國際多中心 Ⅲ 期臨床研究[89]證實了侖伐替尼在晚期肝癌治療效果上不差于索拉非尼,因此,侖伐替尼已獲批晚期肝癌一線治療。此外,瑞戈非尼是晚期肝癌的二線分子靶向藥物。
5.4.2.2 系統化療
奧沙利鉑在我國被批準用于治療不適合手術切除或局部治療的肝癌[95]。適應證主要為:① 合并有肝外轉移的晚期患者;② 雖為局部病變,但不適合手術治療和 TACE 者;③ 合并門靜脈主干或下腔靜脈癌栓者;④ 多次 TACE 后血管阻塞 和(或)TACE 治療后復發的患者。
5.4.2.3 免疫治療
肝癌的免疫治療主要包括免疫調節劑(胸腺肽 α1、干擾素 α 等)[78-79, 83]、腫瘤疫苗(樹突細胞疫苗等)和細胞免疫治療[239],這些治療手段均有一定的抗腫瘤作用,但尚待大規模的臨床研究驗證。近年免疫檢查點阻斷劑(CTLA-4 阻斷劑、PD-1/PD-L1 阻斷劑等)成為研究的熱點,但 PD-1 單抗單藥治療肝細胞癌的臨床療效不盡如人意。免疫聯合治療顯示出了令人鼓舞的應用前景,主要包括免疫治療(PD-1/PD-L1 阻斷劑)聯合抗血管生成治療、雙免疫聯合治療(CTLA-4 阻斷劑聯合 PD-1/PD-L1 阻斷劑)等[240-241]。一項隨機對照、開放標簽的國際多中心 Ⅲ 期臨床研究[242]發現,PD-L1 單抗阿替利珠單抗和貝伐珠單抗聯合治療在療效和安全性方面均優于索拉非尼,成為一線治療的新選擇。國產卡瑞利珠單抗(抗 PD-1 單抗)用于曾接受過肝癌一線治療的晚期肝癌患者,顯示出抗癌作用及可處理的毒性[243],前不久獲批肝癌的二線治療。
5.4.3 中醫中藥
中醫中藥治療能夠改善癥狀,提高機體的抵抗力,減輕抗腫瘤治療的不良反應,提高患者生活質量。我國已批準了現代中藥制劑槐耳顆粒用于肝癌手術切除后的輔助治療(證據等級 1)[244]。其他缺乏高級別的循證醫學證據。
5.4.4 最佳支持治療
適度的康復運動、積極鎮痛、改善睡眠、增加營養、心理治療等對癥支持治療可增強機體的免疫功能。
6 展望
該第二版共識,不少細節增加了高級別循證醫學證據結果,特別是我國學者對肝癌切除術后復發轉移防治不懈努力取得的成果,是按照臨床醫生一接觸患者及在以后診斷治療的每一環節,甚至到肝癌術后復發轉移發生后,都盡量按規范處理病情的思路寫成的,希望能夠作為我們處理肝癌初診患者和復發轉移患者時的參考。同時,肝癌的診斷、治療及肝癌切除術后復發轉移防治相關的很多隨機對照研究正在進行中,我們期望能逐步完善該共識。
在肝癌診治的臨床實踐中應重視以下基本原則:① MDT 是肝癌診治的必要工作方式,按各學科規范為肝癌患者選擇最適合的診斷治療措施,讓患者最大的生存獲益,從而改善肝癌患者的總體預后。對于復雜的復發性轉移性肝癌、需要序貫或聯合治療的肝癌等,MDT 會為患者帶來更多獲益。② 中國的多數肝癌患者無機會進行切除,及切除術后復發轉移發生率高,導致多數患者系帶瘤生存,因此應努力改善他們的心理狀態,提高他們的生命質量。③ 肝癌切除術后復發轉移患者多,病情復雜,通過建立復發肝癌生物樣本庫,加強對肝癌復發轉移的發生發展內在相關分子機制的研究,特別是肝癌的分子分型、靶向治療、免疫治療和相關的轉化醫學的探索,及開展更多的多中心前瞻性隨機對照研究和真實世界研究,來開發和驗證有效的復發性轉移性肝癌診斷治療方法,優化綜合治療措施以改善預后,為精準治療復發性轉移性肝癌提供更多證據,推動我國肝癌防治事業的發展。
《肝細胞肝癌全程多學科規范化管理:華西醫院多學科專家共識》編審成員名單
名譽組長:嚴律南
組 長:文天夫
成員(以姓氏拼音為序):
曹丹5 陳恩強6 陳衛霞2 陳哲宇1 黃紀偉1 蔣利1 李波1 李秋5 李志平5 李川1
魯昌立4 盧強3 盧武勝1 羅燕3 劉非1 唐紅6 王文濤1 王辛5 魏永剛1 吳苾2
吳泓1 徐明清1 楊家印1 楊雨5 曾勇1 張鳴1
(1:肝臟外科;2:放射科;3:超聲診斷科;4:病理科;5:腫瘤中心;6:感染性疾病中心)
編寫秘書:張曉赟 彭偉 劉暢 聶世鴻 沈俊頤 金諶
1 前言
原發性肝癌是目前我國第 4 位常見惡性腫瘤及第 2 位腫瘤死亡病因,嚴重威脅著我國人民的健康和生命[1-2],其中 85%~90% 為肝細胞肝癌(hepatocellular carcinoma,HCC,以下簡稱肝癌)。我國肝癌長期生存率不高的主要原因,首先是肝癌高危人群篩查沒有普及,早期診斷率低,導致 70%~80% 的患者在診斷時已經是中晚期[3-4];其次是多個學科都在診治肝癌,肝癌專科化和規范診治還需加強;再次是,雖然手術切除,包括肝切除術和肝移植術,是肝癌患者獲得長期生存最重要的措施,但肝癌切除術后 5 年復發轉移率高達 40%~70%[4-5]。四川大學華西醫院肝臟外科及相關學科一直力求規范診治肝癌患者,并建立和加強肝癌多學科綜合治療協作組(multidisciplinary team,MDT)工作,最大限度地發揮各個學科的專業優勢,使患者獲益最大化。為了及時反映近 3 年肝癌研究的最新成果,四川大學華西醫院肝癌 MDT 團隊,對 2017 年版《肝細胞癌切除術后復發轉移的防治:華西醫院多學科專家共識》 [5-6]進行了修訂,以規范和推進肝癌全程多學科規范化管理的新模式。
本共識中的證據等級分為 6 級,推薦意見為 5 級[7-8],見表 1 和表 2。
下載CSV
| 證據級別 | 描述 |
| Ⅰa | 證據源于對多項隨機對照研究的 Meta 分析結果 |
| Ⅰb | 證據源于至少 1 項設計良好的隨機對照研究結果 |
| Ⅱa | 證據源于至少 1 項設計良好的前瞻性非隨機對照研究結果 |
| Ⅱb | 證據源自至少 1 項設計良好的其他類型干預性臨床研究結果 |
| Ⅲ | 證據源于設計良好的非干預性研究,如描述性研究、相關性研究等 |
| Ⅳ | 證據源于專家委員會報告或權威專家的臨床經驗報道 |
下載CSV
| 證據等級 | 描述 |
| A | 良好的科學證據提示該醫療行為帶來明確獲益,建議醫師對患者實施該醫療行為 |
| B | 現有證據表明該醫療行為可帶來中度獲益,超過其潛在風險;醫師可建議對患者實施該醫療行為 |
| C | 現有證據表明該醫療行為可能獲益較小,或獲益與風險接近;醫師可根據患者個體情況有選擇地向患者建議或實施該醫療行為 |
| D | 現有證據表明該醫療行為無獲益,或其潛在風險超過獲益;醫師不宜向患者實施該醫療行為 |
| E | 缺乏科學證據,或現有證據無法評價該醫療行為的獲益與風險;醫師應幫助患者理解該醫療行為存在的不確定性 |
2 從肝臟占位性病變初診到診斷肝癌
2.1 肝癌的診斷標準
2.1.1 臨床診斷標準
國際上公認,可以采用臨床診斷標準的實體瘤,唯有肝癌[3-4, 9]。臨床診斷肝癌主要依據 3 個方面:即慢性肝病背景、影像學特征及血清 AFP 水平。因肝癌巨大的異質性,影像學表現復雜多樣、AFP 水平差異較大,實際應用時需綜合分析、嚴格掌握標準。目前國際上公認的臨床診斷標準為:同時滿足以下條件中的 2.1.1.1+2.1.1.2.1+2.1.1.3 三項或 2.1.1.1+2.1.1.2.2 兩項;否則,應進一步穿刺活檢[4, 6, 9-10]。
2.1.1.1 肝病史
具有肝硬變以及 HBV 和(或)HCV 感染 [HBV 和(或)HCV 抗原陽性] 的證據,或非酒精性脂肪肝病史[10]
2.1.1.2 典型的肝癌影像學特征
MRI 和(或)CT 動脈增強掃描或增強多期掃描顯示肝內病灶在動脈期不均勻或均勻強化,即富血供(arterial hypervascularity),而門靜脈期或延遲期快速洗脫(venous or delayed phase washout)。MRI 肝膽特異性對比劑釓塞酸二鈉增強掃描,動脈期均勻或不均勻強化、門靜脈期無快速洗脫,但肝膽期無強化;或動脈期無強化,門靜脈期無強化或輕度強化,肝膽期無強化,且病灶在高 b 值 DWI 圖像呈高信號[11-12]。美國放射學會開發的肝臟影像報告和數據系統(liver imaging reporting and data system,LI-RADS)對肝癌和復發性肝癌(recurrent HCC,RHCC)影像學的解讀有一定幫助[13]。
2.1.1.2.1
如果肝臟病灶直徑為 1~2 cm,需要 CT 和 MRI 兩項檢查都顯示具有肝癌典型影像學特征,可診斷肝癌,以加強診斷的特異性。
2.1.1.2.2
如果肝臟病灶直徑>2 cm,CT 和 MRI 兩項檢查中有一項顯示肝臟占位具有肝癌典型影像學特征,即可診斷肝癌。
2.1.1.3 血清 AFP
AFP水平≥400 μg/L 持續 1 個月或≥200 μg/L 持續 2 個月,并能排除其他原因引起的 AFP 升高,包括妊娠、生殖系胚胎源性腫瘤、活動性肝病、消化道腫瘤等。GALAD 和 BALAD 評分系統,主要利用肝癌相關標志物 AFP、AFP-L3、異常凝血酶原(des-γ-carboxy prothrombin,DCP)水平等構建的數學模型,可提高早期肝癌的檢出率,越來越受到歡迎[14-15]。
2.1.2 病理學診斷標準
肝臟占位病變或者肝外轉移灶的穿刺活檢組織或手術切除標本,經細胞學和(或)病理組織學檢查診斷為肝癌,此為診斷的金標準。肝癌的病理學診斷規范由標本處理、標本取材、病理學檢查、病理報告等部分組成,對手術切除標本,推薦采用“7 點”基線取材法和病理報告的結構化報告格式,盡可能反映出肝癌復發轉移的高危因素[16]。
2.1.3 RHCC 的診斷標準
與初次肝癌診斷標準相同,甚至要求兩種影像學檢查顯示肝癌的血供特征[4, 17-20]。兩種甚至三種影像學檢查可優勢互補[4],對準確的 RHCC 預后預測和幫助選擇最佳治療方式有積極意義。在慢性肝病背景下,肝內實性病灶的定性,推薦采用 MRI 肝膽特異性對比劑增強掃描,對鑒別治療后壞死灶、出血灶、再生結節與 RHCC,是目前國際上公認的準確的影像學檢查方法。在 RHCC 診斷明確,進入治療之前,或未診斷 RHCC 而 AFP 升高、或伴有癌轉移其他臨床表現的患者,應進行胸部 CT 和全身骨顯像甚至 PET-CT 檢查,以診斷轉移性肝癌(metastatic HCC,MHCC) [4, 20]。復發性轉移性肝癌診斷路徑見圖 1。
2.1.4 肝占位病變初診患者的檢查評估目標
肝癌患者通常在多年慢性乙肝感染或(和)慢性丙肝感染的基礎上發生肝硬變,進而可發生肝癌[4],有的患者其肝臟還伴有肝囊腫和(或)血管瘤等,那么通過病史、乙肝及丙肝標志物檢查、腫瘤標志物檢查、影像學檢查等綜合判斷的目標,就是明確患者肝臟占位性病變中有沒有肝癌及有幾個肝癌病灶。
2.2 不能切除肝癌的轉化切除
對初診不能夠切除的肝癌進行介入治療、靶向治療、化療、放射治療和免疫治療,其中達到部分緩解和完全緩解的患者,再次評估后可進行第二步切除,也稱降期切除,現統稱為轉化切除[21]。轉化切除是提高肝癌切除率的重要途徑,是不能夠切除肝癌經過綜合治療后達到的最好目標。轉化治療的途徑包括以增加剩余肝體積為主要目標的肝癌轉化切除,包括門靜脈栓塞(portal vein embolization,PVE) [22]、聯合肝臟離斷和門靜脈結扎的二步肝切除術(associating liver partition and portal vein ligation for staged hepatectomy,ALPPS) [23-25]、肝靜脈系統栓堵術(liver venous deprivation,LVD) [26-27]等措施;以縮小肝癌為主要目標的轉化切除,包括肝動脈化療栓塞(transcatheter arterial chemoembolization,TACE) [28]、系統治療(靶向治療、免疫治療和化療) [29-30]、放療等方式;以促進剩余肝增生和縮小肝癌兩個維度[31]都實施的轉化治療等多種方法,值得深入研究。
2.3 可切除肝癌術前對預后的預測和考慮
2.3.1 肝癌肝切除術前對復發高危風險的預測
肝癌肝切除術后復發高危風險是指伴血管癌栓、微血管侵犯(microvascular invasion,MVI)和多結節肝癌,中危風險是指肝癌直徑大于 5 cm[32]。大體病理觀察和影像學檢查顯示,單結節肝癌的預后好于多結節融合型肝癌[33-35]。隨著肝癌的增大,發生 MVI 的可能性逐漸增加[36]。有研究[37]顯示,肝癌病灶大小(直徑截點值 3.6 cm)、DCP(截點值 101 mAU/mL)和最大標準化攝取值(the maximum standardized uptake value,SUVmax,截點值 4.2)構建的評分系統,預測 MVI 的敏感度與特異度可達 100% 和 90.9%。Lee 等[38]用釓塞酸二鈉增強 MRI 術前預測單個肝癌伴 MVI 的研究顯示:動脈期的腫瘤邊周強化、腫瘤邊緣不光滑和肝膽期腫瘤周邊低增強等三項或其中的兩項,可作為術前預測 MVI 的影像學特征,其特異度超過 90%。沈鋒團隊[39]用 AFP 水平、HBV-DNA 水平、血小板計數、腫瘤影像學特征、影像學腫瘤個數、影像學腫瘤大小和影像學腫瘤包膜 7 項指標創作列線圖,用于術前預測乙肝相關性 Milan 標準肝癌伴發 MVI 的研究顯示:積分大于 200 屬于伴 MVI 高風險組,其陽性預測值達 57.4%。術前影像學檢查顯示 4 個及以上的多結節肝癌不首選手術切除[4]。Li 等[40]發現,多結節肝癌無論符合 Milan 標準與否,肝移植的預后優于肝切除。Li 等[41]發現門靜脈主干癌栓或 3 個結節肝癌伴 AFP 高于 400 μg/L 者行肝切除治療為無益肝切除。門靜脈癌栓和(或)淋巴結轉移患者不適合接受肝移植[42-43]。肝癌干細胞標志物高表達[44]和表達上皮細胞黏附分子(epithelial cell adhesion molecule,EpCAM)的循環腫瘤細胞(circulating tumor cells,CTC,EpCAM+CTC7.5)≥2[45]提示預后不良。
若預測到某患者存在 MVI,需怎么做呢?目前我們可能應改進原來的處理思路,不宜對該患者推薦射頻治療,該患者即便行肝移植也易復發,可考慮行解剖性肝切除和(或)寬切緣切除術及術后輔助治療,也可考慮行新輔助治療以改善預后[39, 46]。
2.3.2 肝癌的新輔助治療
因為介入技術與放療技術的進步和藥物的涌現,在肝癌可切除但預后不佳的患者中,已初步顯現術前新輔助治療可以改善預后,讓患者更多獲益。Allard 等[47]報道,對 TACE 有病理學反應的肝癌患者在肝切除或肝移植后的預后好于無或較差病理學反應的肝癌患者。程樹群團隊[48]報道的一項多中心 RCT 顯示:術前加用三維適形放療可明顯改善肝癌伴 PVTT 患者行肝切除術后的無復發生存率(RFS)和總體生存率(OS)。
3 肝癌切除術中預防復發轉移的措施
右肝大肝癌,特別是影像學檢查提示膈肌受侵者,應行前入路肝切除[49-50]。如預估斷肝出血達到 600~800 mL,應行入肝血流阻斷或半入肝血流阻斷[51-52]。術中大量出血、輸血會影響患者的預后[53];而入肝血流阻斷不會影響腫瘤患者的預后[54]。肝癌手術中超聲甚至超聲造影[55]可進一步顯示癌旁有無衛星結節、癌栓、余肝有無結節、病灶與第一、第二和第三肝門的關系,并幫助確定切線。ICG 熒光技術也可發現衛星結節、幫助確定解剖性肝切除的切線等[56]。如術中發現明確或可疑病灶,應考慮同時切除或消融治療[57-58]。根據腫瘤范圍、肝硬變程度、肝功能與肝儲備功能、預測的殘肝體積等應依次選擇解剖性肝切除、或寬切緣非規則性肝切除、或腫瘤局部切除術,以盡可能切除癌周可能存在的 MVI 和衛星結節[46, 59-60]。
4 肝癌切除術后預防復發轉移的措施和定期隨訪
4.1 肝癌根治性切除標準[3 -5 , 18 ]
4.1.1 術中判斷標準
① 肝靜脈、門靜脈、膽管以及下腔靜脈未見肉眼癌栓。② 無鄰近臟器侵犯,無肝門淋巴結或遠處轉移。③ 腫瘤切緣>1 cm;若切緣<1 cm,但切除肝斷面組織學檢查無腫瘤細胞殘留,即切緣陰性。④ 術中超聲和 ICG 熒光技術未發現有衛星灶、小脈管癌栓、新病灶等。
4.1.2 術后病理報告判斷標準
規范性病理取材的病理報告[16]未報 MVI、衛星結節、切緣陽性等。
4.1.3 術后 2 個月判斷標準
① 術后 2 個月行超聲、CT、MRI(必須有其中兩項)檢查未發現腫瘤病灶;② 如術前 AFP 和(或)DCP 升高,則要求術后 2 個月行 AFP 和(或)DCP 定量測定,其水平在正常范圍(極個別患者 AFP 降至正常的時間超過 2 個月)。
4.2 肝癌切除術后伴有復發轉移高危因素患者的預測、監測與治療
4.2.1 肝癌切除術后復發轉移的高危因素
肝癌切除術后 1~2 個月,應結合術中情況、AFP 和 DCP 下降情況及病理報告,再次評估患者的復發轉移高危因素,制定應實施的輔助治療,甚至作出預后基本預測。若血小板淋巴細胞比(PLR)>107、MVI 陽性和單個肝癌病灶直徑≥6.8 cm 3 個因素同時存在,提示預后差[61]。導致肝癌術后復發轉移的危險因素很多:① 手術因素,包括非解剖型肝切除[62]、窄切緣[60]、手術切緣腫瘤殘留[63]、較大量出血輸血[64]、術中擠壓腫瘤[49]等;② 臨床病理因素,包括腫瘤低分化、腫瘤分期較晚[4]、腫瘤破裂[65]、無完整包膜、腫瘤直徑大>5 cm、腫瘤數目≥3 個[66-67]、脈管侵犯[46]、淋巴結轉移[68]、衛星灶、鄰近器官受侵[4]、AFP 明顯升高[67]、AFP 術后 2 個月未降至正常水平[69]、術后血管造影見殘存陽性病灶等;③ 背景肝病因素,包括肝炎病毒感染[70]、肝硬變等。
4.2.2 肝癌切除術后伴有復發轉移高危因素患者的治療
對于肝癌切除術后伴有復發轉移高危因素的患者,目前認為應考慮輔助治療[4-6, 71],以預防腫瘤復發。已有較多研究[32, 71-77]顯示,對伴有明確復發轉移風險如 MVI、多結節和腫瘤直徑>5 cm 的肝癌切除術后患者,術后輔助 TACE 治療,可使患者生存獲益。對伴有復發轉移高危因素患者根據情況還可進行聯合治療,包括胸腺肽 α1[78-80]或 α-干擾素[81-85]、索拉非尼[86-88]或侖伐替尼[89-90]、維生素 K2[91-94],還可考慮聯合化療[95-96]等。
4.2.3 肝癌切除術后患者的定期隨訪
目前證據表明,對于沒有復發轉移中高危因素的患者采取不恰當的輔助治療如 TACE,可能引起殘余肝臟的損害,導致肝功能惡化而致生活質量下降,反而影響長期生存,甚至可使肝外轉移的發生率升高,預后更差[72, 77]。乙肝或丙肝相關性肝癌,根據情況需要行抗病毒治療[70, 97-99]。
肝癌患者術后應進行定期隨訪[4-6, 71, 100],內容主要包括[4, 6, 71]肝臟影像學檢查、肝癌標志物(AFP 和 DCP)檢查以及肝功能檢查,隨訪頻度在 2 年內是每 3~4 個月隨訪 1 次,以后則可適當延長。HBV-DNA 的復查根據肝功能情況、是否抗病毒治療等進行選擇。
5 肝癌切除術后復發和轉移
肝癌病情的進展多見于肝內,如肝內病灶長大、門靜脈侵犯、術后復發等,而遠處轉移相對較少,約 13.5%~42%[101-103]。
5.1 肝癌切除術后復發模式及臨床意義
目前廣泛認可的 RHCC 來源于:① 肝癌切除后,肉眼難以查見的殘留腫瘤細胞繼續生長或通過肝內血運播散形成的肝內轉移(intrahepatic metastasis,IM);② 由于 HBV 或 HCV 感染,肝臟在長期慢性炎癥反應及肝硬變背景下,正常肝細胞染色體長期累積突變而發生的惡性轉化,形成多中心發生(multicentric occurrence,MO)的 RHCC。
最早的鑒別方法是基于臨床病理資料的總結,根據 RHCC 復發時間,將 1 年內復發的 RHCC 定為 IM,是為早期復發,1 年以上復發的 RHCC 定為 MO,是為晚期復發[104-105]。MO 的總體生存率好于 IM。也有基于病理診斷判定 IM 的標準[106]以及基于腫瘤的分化情況提出 MO 的鑒別方法[107-108],但其敏感度和特異度均不理想,很大程度受到病理醫生主觀因素的影響。隨著分子生物學技術及基因組學技術的發展,臨床及病理學者對 RHCC 來源探索了比較多的鑒別方法,包括微衛星雜合性缺失模式(loss of heterozygosity,LOH)、微衛星不穩定性檢測(microsatellite instability)、p53 基因點突變模式分析(TP53 gene mutation analysis)、X 染色體失活性分析(X chromosome inactivation analysis)、HBV-DNA 整合位點檢測(HBV-DNA integration detection)、DNA 甲基化模式檢測(DNA methylation analysis)、miRNA 譜試分析、比較基因組雜交分析(comparative genomic hybridization,CGH)等[109-118]。有學者[119-120]綜合多組學的方法并聯合臨床病理資料,鑒定多結節 HCC 的發生來源及腫瘤異質性。其中 LOH 運用廣泛。微衛星 DNA 是反應細胞 DNA 整體穩定性的良好標志物,聯合多個高頻 LOH 染色體能提高鑒別 RHCC 的準確性。所需標本條件較易達到,甲醛固定石蠟包埋的樣本或穿刺標本都可滿足檢測要求[109]。
肝癌的復發轉移時間極其混亂[104-105, 121-123],從復發肝癌患者手術切除的標本進行克隆分析顯示[112],“復發”腫瘤有來自原發癌的,也有為新生腫瘤的。臨床上,以復發時間 1 年為界判斷為早期復發還是晚期復發,符合絕大多數患者的肝癌復發情況[6, 104-105],簡單實用。原則上,對于 1 年內復發的肝癌即 IM,有時貌似可以切除,但應選擇消融治療、放療、TACE 聯合靶向治療等微創治療手段;而對于 1 年以上復發的肝癌即 MO,可選擇再切除或肝移植等根治性治療措施[6,112]。
5.2 復發性肝癌的治療
5.2.1 復發性肝癌治療路線圖
目前已經明確,初治肝癌為多個腫瘤、伴 MVI 等,是肝癌切除術后的復發高危因素;同時,初次肝癌手術切除 1 年以內還是 1 年以上復發,治療方式選擇的原則不同。我們基于這個原則,并參考原發性肝癌診療規范(2017 年版) [3]建立了一個初步的 RHCC 治療流程[6],見圖 2。
5.2.2 復發性肝癌再切除
5.2.2.1 復發性肝癌再切除
有研究[124-125]顯示,肝切除術后的可切除性復發肝癌,再次肝切除者的總生存率(OS)優于 TACE 治療者;系統評價也顯示出了相同的結論[126];甚至有研究[127-128]認為,患者能夠從第 3 次手術切除中生存獲益;而第 4 次肝切除并不會改善患者的生存情況[129]。對于可切除的肝外轉移灶,手術切除同樣能夠給患者帶來生存獲益[130]。復發肝癌患者再次手術的預后與患者初次手術時的臨床病理特征、復發間隔時間等密切相關[131]。再次切除患者最好是初次手術時腫瘤單發、腫瘤復發時間間隔≥1 年,而且復發時腫瘤無血管侵犯[6, 132]。復發肝癌患者再次的術前評估與初次術前評估一樣,應該考慮患者的體能狀態、肝功能、肝儲備功能、肝硬變程度、門靜脈高壓程度、剩余肝臟體積等[127, 133-135]。再次肝切除要求患者的 ECOG PS 評分為 0~1 分,應無明顯的心、肺、腦、腎等重要器官功能障礙;肝功能 Child-Pugh A 級或者肝功能 B 級經短期護肝治療后恢復到 A 級;肝臟儲備功能良好。患者的剩余肝臟體積(FLV)應結合患者的肝功能、肝臟儲備功能等多項指標綜合考慮[135]。對肝硬變、Child-Pugh A 級、ICG R15<10% 的患者,FLV 應>40%;而 ICG R15 為 10%~20% 的患者,FLV 應>50%[136-138];對于肝纖維化的患者,FLV 應>30%[139];而對于正常肝臟患者,FLV 應>20%[139]。高齡患者經過嚴格評估后,行再次肝切除同樣是安全可行的[140]。
5.2.2.2 可切除性復發肝癌的腹腔鏡手術
與傳統手術相比,腹腔鏡肝切除手術具有創傷小、術后恢復快等優點。許多研究亦證實,對于復發肝癌患者,腹腔鏡手術后無瘤生存率及總體生存率與傳統開腹手術無明顯差異[141-145]。但值得注意的是,這些研究中的許多腹腔鏡手術,對患者的腫瘤大小和位置等已有選擇[142-143, 146]。近年一項多中心研究證實,對于腹腔鏡肝切除術中中轉開腹的患者術中出血量、輸血量、術后住院時間、并發癥發生率和死亡率均明顯增加[147]。
5.2.2.3 可切除性復發性肝癌伴門靜脈癌栓的手術適應證
肝癌合并門靜脈癌栓是否應接受手術切除一直存在較大爭議,近年來有較多文獻報道此類患者中的部分患者可從手術中獲益。亞太地區學者認為對于肝癌合并門靜脈癌栓的部分患者應考慮手術切除,但應重視患者篩選[42, 148-149]。對于復發肝癌伴門靜脈癌栓,更應嚴格把握此類患者的手術切除適應證。首次根治性切除術后復發時間大于 1 年,為可切除性復發腫瘤,門靜脈癌栓程氏分型屬于Ⅰ、Ⅱ型,適合肝段、肝葉等解剖性肝切除,可考慮行肝葉、半肝等解剖性肝切除并整塊切除癌栓[42]。但肝癌合并Ⅲ型門靜脈癌栓患者預后差,目前基本不首選手術切除[40, 42]。
5.2.2.4 可切除性復發性肝癌伴膽管癌栓的手術適應證
初次手術切除肝癌不伴 MVI 等復發高危因素、復發時間 1 年以上的肝癌肝內復發伴膽管癌栓,原則上應爭取外科手術完整切除腫瘤及取盡癌栓,特別是對那些不伴梗阻性黃疸的患者[150-154]。腫瘤個數小于 3 個、局限在肝段或肝葉、預估有足夠的殘余肝體積、癌栓位于腫瘤側膽管,可行腫瘤及癌栓累及膽管的解剖性肝切除。復發肝癌可切除,癌栓進入膽總管,癌栓形成時間較短尚未發生機化,可行肝葉、半肝等的解剖性肝切除加膽管癌栓取出術加 T 管引流術[152-153, 155];如癌栓形成時間長、癌栓可能侵及膽管壁但局限在肝外膽管、復發肝癌可切除,可考慮行解剖性肝切除及肝尾葉切除加肝外膽管切除及膽腸吻合術[17, 150, 156-157]。
5.2.2.5 可切除性復發性肝癌伴肝硬變、門靜脈高壓癥、脾大及脾功能亢進聯合脾切除術適應證
復發性肝癌具有可切除性,伴中、重度脾臟腫大,重度脾功能亢進(WBC <3×109/L,PLT <50×109/L)表現者,應該考慮行肝癌切除聯合脾切除[158-163]。有明顯食管胃底靜脈曲張,特別是發生過食管胃底曲張靜脈破裂大出血患者,經過嚴格的評估后還應同時行賁門周圍血管離斷術。對有嚴重胃黏膜病變者,如患者術中情況允許,應行脾腎分流術或者其他類型的選擇性門腔分流術。此類患者都應考慮到將來有行肝移植的可能性,因此不宜行影響第一肝門的分流術。此類聯合手術宜從嚴掌握,如果患者既往有肝功能不全、凝血功能障礙等病史,應考慮行挽救性肝移植。
5.2.3 復發性肝癌消融治療
局部消融治療的消融方式包括:射頻消融(RFA)、微波消融(MWA)、高功率超聲聚焦消融(HIFU)、冷凍治療(CRA)、無水乙醇注射治療(PEI)等[164-166]。
較多研究[167-171]顯示,肝癌切除術后復發肝癌患者可以從 RFA 中獲益,甚至可取得與再切除相近的 OS 及 DFS。與切除相比,射頻消融的明顯優勢在于微創、恢復快、并發癥少、可重復實施性強,還可忽略首次手術時是否存在復發高危因素或復發時間。對于復發肝癌,單個腫瘤直徑≤5 cm;腫瘤數目≤3 個、其最大直徑≤3 cm;不伴有脈管內癌栓或鄰近器官受侵;超聲顯示有射頻路徑,腫瘤可達到完全消融,是射頻消融治療的指征[172-175]。對直徑<2 cm 的肝癌,RFA 作為一線治療[10]。需要特別注意的是,對于直徑>3 cm 的腫瘤,射頻消融中應采用多點覆蓋,以確保病灶完全消融[176],或選擇 MWA 消融[177-182]。
有較多研究[183-184]比較了 CRA、RFA 和 MWA 治療直徑<5 cm 肝癌的療效,結果發現,上述 3 種治療的 OS 和 DFS 無差異;但對于直徑為 3~4 cm 的腫瘤,CRA 術后局部復發率較 RFA 更低。有研究提示 HIFU 和 RFA 對于符合米蘭標準的復發性肝癌治療效果相當[185];而 PEI 對直徑≤2 cm 的肝癌消融效果確切[186-189]。
5.2.4 復發性肝癌行肝移植
許多研究[190-193]報道,對于復發性肝癌行挽救性肝移植可以生存獲益。甚至有研究[191-192]認為,復發性肝癌行挽救性肝移植,OS 明顯優于行再次手術切除。在不少研究中,初次手術時的腫瘤符合米蘭標準[191, 193-195],也有初次手術時腫瘤超過米蘭標準[196-197],但多數研究中,患者初次手術時均無大血管癌栓。在行挽救性肝移植時,大多數中心要求腫瘤情況符合米蘭標準[190-191, 194-195, 198],但有些中心采用擴展標準,如京都大學標準[199]、九州大學標準[192]、杭州標準[196]、up-to-7 標準[197]。許多研究[190, 192, 199]亦證實,對于復發肝癌患者,活體肝移植是安全有效的。但目前尚缺乏針對復發肝癌患者尸體肝移植和活體肝移植預后比較的報道。
有研究[200-202]顯示,肝癌切除術后發現有復發高危因素時就登記肝移植,即預防性肝移植,可能比挽救性肝移植效果更好。但需更多的研究數據證實。
5.2.5 復發性肝癌的 TACE 治療
早期的 RCT 研究[203-204]顯示,局限在肝內且沒有血管侵襲的中晚期肝癌患者可從 TACE 治療中獲益。TACE 序貫治療,其中約 10% 的肝癌患者可以達到完全緩解[205]。肝癌負荷大小是決定 TACE 治療效果的主要因素,肝癌負荷小的,TACE 效果好[206]。對于復發性肝癌數目≤3 個,直徑≤5 cm 時,TACE 和 RFA 的效果相當[207],尤其是對于切除或移植術后多發肝內復發的患者,TACE 是降低復發后死亡率的重要手段[208]。還有研究[209]顯示,符合米蘭標準的復發肝癌,早期復發時 TACE 和 RFA/切除具有相同效果,晚期復發時 RFA/切除效果好于 TACE。Jin 等[209] 比較了 TACE、切除和 RFA 對伴 MVI 的復發肝癌的治療效果,結果顯示 TACE 治療 MVI 陽性復發肝癌可獲得較切除和 RFA 更好的 OS 和 DFS,特別對于 1 年內復發的 MVI 陽性者,OS 顯著高于切除和 RFA 組。Yang 等[210]回顧性研究了 TACE 聯合 RFA 與單獨行 TACE 或 RFA 治療復發性肝癌的效果,聯合治療組的 5 年生存率顯著高于單獨 TACE 或 RFA 組。TACE 治療復發肝癌的指征[210-211]:① 復發性肝癌臨近重要血管或膽管而無法行切除或 RFA 術;② 肝內多發復發性腫瘤;③ 早期肝內復發性肝癌(1 年內);④ 患者個人愿意選擇 TACE 治療。
5.2.6 復發性肝癌的放射治療
放射治療(簡稱放療)分為外放療和內放療。外放療是利用放療設備產生的射線(光子或粒子)從體外對腫瘤照射。內放療是利用放射性核素,經機體管道或通過針道植入腫瘤內[4]。
5.2.6.1 肝癌外放療技術[4 ]
包括三維適形或調強放療、圖像引導放療(image guided radiation therapy,IGRT)或立體定向放療(stereotactic body radiation therapy,SBRT)等技術。外放療已經成為治療復發性肝癌有效和安全的手段之一[212-214],國內外研究報道小肝癌行放療 5 年生存率可達 60% 以上[213-215],甚至療效等同于 RFA 治療[216],但與手術相比尚無直接證據。復發性肝癌患者常面臨腫瘤位于切除困難位置、多發、伴大血管癌栓及肝功能失代償等,也常伴發肺、骨等部位的肝外轉移,可根據腫瘤的情況選擇合適的放療方案,可多次實施以縮小肝內外病灶,控制腫瘤進展,減輕腫瘤引起的癥狀,以延長患者的生存時間。如肺轉移患者經體外放射治療 2 年生存率可達 70.7%,骨轉移患者經體外放射治療中位生存期達到 7.4 個月[217-221]。近年來,對于肝癌局部明顯進展如伴門靜脈癌栓和肝靜脈/腔靜脈癌栓,加用外放療取得了較明顯療效[48, 222]。
5.2.6.2 內放療技術[4 , 223 ]
即放射性粒子植入技術,包括組織間植入、門靜脈植入、下腔靜脈植入和膽管內植入,分別治療肝內病灶、門靜脈癌栓、下腔靜脈癌栓和膽管內癌或癌栓。放射性粒子包括90Y 微球、131I 單克隆抗體、放射性碘化油、125I 粒子植入等。已有動脈灌注90Y 微球治療肝癌肝移植術后復發,可有效控制復發癌進展的報道[224]。氯化鍶(89Sr)發射出 β 射線,可用于靶向治療肝癌骨轉移病灶[225]。肝癌患者接受 TACE 放射微球[226]治療能獲得更高的腫瘤壞死率和更低的腫瘤進展率。但其在復發性肝癌中的作用尚待研究。
5.2.7 復發性肝癌的聯合或序貫治療
在中國,目前肝癌診治領域的特點仍然是多方法、多學科共存,而以治療手段建科的分科診療體制與實現有序規范的肝癌治療之間存在一定的矛盾[3],因此肝癌 MDT 下規范治療的選擇與綜合治療極其重要,特別是對復發性肝癌的治療。復發肝癌切除聯合術中射頻治療,已進行抗病毒治療的復發肝癌切除術后伴有復發轉移高危風險患者再序貫 TACE、靶向治療與胸腺肽 α1 治療,及肝內復發肝癌 RFA 序貫 TACE 治療[211, 227]等,都是聯合或序貫治療的基本模式,能夠降低腫瘤負荷,延長患者生存。
5.3 肝癌切除術后肝外轉移
肝癌病情進展多見于肝內,而遠處轉移相對較少,為 13.5%~42%[101-103]。肝癌患者死亡原因也更多為肝功能衰竭和肝內腫瘤引起的其他相關并發癥。肝癌常見的肝外轉移部位是肺,其次是淋巴結、骨、腎上腺、腹膜等,也可直接侵犯臨近器官[101, 103]。
肝癌切除術后肝外轉移的發生可概括為 3 種模式[103]:① 先肝內復發后肝外轉移;② 同時性肝內復發肝外轉移;③ 首先發生肝外轉移。Yang 等[103]對 348 例術后患者隨訪(4.8±3.7)年,發現肝內復發、肝外轉移分別為 64% 和 14%。3 種肝外轉移模式中,患者發生肝外轉移后總生存時間并無明顯差異,但先肝內復發后肝外轉移模式中的肝外轉移出現時間最晚,分別為(3.2±0.8)年、(0.8±0.5)年和(0.9±0.2)年。
盡管肝癌切除術后復發轉移以肝內復發多見,但術后隨訪復查不能忽視肝外轉移的可能性。結合 AFP、DCP、彩超、CT、MRI 等常規檢查手段,早期發現肝內復發和(或)肝外轉移,避免漏診,才能為后續治療提供準確依據。18F-FDG PET 或 PET/CT 對于腫瘤診斷是很好的功能成像技術,然而對于肝癌特別是分化良好的肝癌,其敏感度相對較低。Meta 分析顯示 18F-FDG PET 或 PET/CT 對于肝癌復發轉移診斷的敏感度為僅為 64%,特異度為 95%[228-229]。因此,18F-FDG PET 或 PET/CT 并不作為肝癌診斷或術后隨訪的常規影像學檢查手段,但仍有助于發現隱匿的復發轉移病灶。
對于肝癌切除術后肝外轉移,主要考慮系統治療[89, 95, 230-231]。關于肝外轉移的局部治療,目前沒有高級別的循證醫學證據。但回顧性研究[232-234]顯示,手術、放療、消融等局部治療對部分腫瘤進展緩慢、轉移病灶少的高選擇性肝外轉移患者,可能帶來生存獲益,其回顧性的小樣本研究顯示:術前無病生存期>12 個月、肺轉移灶≤4 個、單純肺轉移、最大病灶直徑<3 cm 等可能與肺轉移術后預后相關。對于肝癌切除術后 1 年以上、單發淋巴結轉移或單純腎上腺轉移的患者,手術切除也可能獲得較長期生存[235-236]。骨轉移比肺轉移預后差,手術切除需謹慎,可考慮放療緩解癥狀,減緩病灶進展[225, 237-238]。
5.4 肝癌切除術后復發轉移的全身治療
5.4.1 抗病毒治療
TACE、手術和化療均可引起乙型肝炎病毒復燃,而且抗病毒治療可以降低肝癌切除術后復發率,改善患者的生存,因此,合并有乙肝病毒感染且復制活躍的肝癌患者,推薦口服核苷(酸)類似物抗病毒治療[70, 98-99]。
5.4.2 肝癌藥物治療
包括分子靶向治療、系統化療和免疫治療。
5.4.2.1 分子靶向治療
索拉非尼是一線治療晚期 HCC 的分子靶向藥物。有研究[86-88]顯示:索拉非尼作為輔助治療,對術后高危復發的患者也有一定的療效。一項大型國際多中心 Ⅲ 期臨床研究[89]證實了侖伐替尼在晚期肝癌治療效果上不差于索拉非尼,因此,侖伐替尼已獲批晚期肝癌一線治療。此外,瑞戈非尼是晚期肝癌的二線分子靶向藥物。
5.4.2.2 系統化療
奧沙利鉑在我國被批準用于治療不適合手術切除或局部治療的肝癌[95]。適應證主要為:① 合并有肝外轉移的晚期患者;② 雖為局部病變,但不適合手術治療和 TACE 者;③ 合并門靜脈主干或下腔靜脈癌栓者;④ 多次 TACE 后血管阻塞 和(或)TACE 治療后復發的患者。
5.4.2.3 免疫治療
肝癌的免疫治療主要包括免疫調節劑(胸腺肽 α1、干擾素 α 等)[78-79, 83]、腫瘤疫苗(樹突細胞疫苗等)和細胞免疫治療[239],這些治療手段均有一定的抗腫瘤作用,但尚待大規模的臨床研究驗證。近年免疫檢查點阻斷劑(CTLA-4 阻斷劑、PD-1/PD-L1 阻斷劑等)成為研究的熱點,但 PD-1 單抗單藥治療肝細胞癌的臨床療效不盡如人意。免疫聯合治療顯示出了令人鼓舞的應用前景,主要包括免疫治療(PD-1/PD-L1 阻斷劑)聯合抗血管生成治療、雙免疫聯合治療(CTLA-4 阻斷劑聯合 PD-1/PD-L1 阻斷劑)等[240-241]。一項隨機對照、開放標簽的國際多中心 Ⅲ 期臨床研究[242]發現,PD-L1 單抗阿替利珠單抗和貝伐珠單抗聯合治療在療效和安全性方面均優于索拉非尼,成為一線治療的新選擇。國產卡瑞利珠單抗(抗 PD-1 單抗)用于曾接受過肝癌一線治療的晚期肝癌患者,顯示出抗癌作用及可處理的毒性[243],前不久獲批肝癌的二線治療。
5.4.3 中醫中藥
中醫中藥治療能夠改善癥狀,提高機體的抵抗力,減輕抗腫瘤治療的不良反應,提高患者生活質量。我國已批準了現代中藥制劑槐耳顆粒用于肝癌手術切除后的輔助治療(證據等級 1)[244]。其他缺乏高級別的循證醫學證據。
5.4.4 最佳支持治療
適度的康復運動、積極鎮痛、改善睡眠、增加營養、心理治療等對癥支持治療可增強機體的免疫功能。
6 展望
該第二版共識,不少細節增加了高級別循證醫學證據結果,特別是我國學者對肝癌切除術后復發轉移防治不懈努力取得的成果,是按照臨床醫生一接觸患者及在以后診斷治療的每一環節,甚至到肝癌術后復發轉移發生后,都盡量按規范處理病情的思路寫成的,希望能夠作為我們處理肝癌初診患者和復發轉移患者時的參考。同時,肝癌的診斷、治療及肝癌切除術后復發轉移防治相關的很多隨機對照研究正在進行中,我們期望能逐步完善該共識。
在肝癌診治的臨床實踐中應重視以下基本原則:① MDT 是肝癌診治的必要工作方式,按各學科規范為肝癌患者選擇最適合的診斷治療措施,讓患者最大的生存獲益,從而改善肝癌患者的總體預后。對于復雜的復發性轉移性肝癌、需要序貫或聯合治療的肝癌等,MDT 會為患者帶來更多獲益。② 中國的多數肝癌患者無機會進行切除,及切除術后復發轉移發生率高,導致多數患者系帶瘤生存,因此應努力改善他們的心理狀態,提高他們的生命質量。③ 肝癌切除術后復發轉移患者多,病情復雜,通過建立復發肝癌生物樣本庫,加強對肝癌復發轉移的發生發展內在相關分子機制的研究,特別是肝癌的分子分型、靶向治療、免疫治療和相關的轉化醫學的探索,及開展更多的多中心前瞻性隨機對照研究和真實世界研究,來開發和驗證有效的復發性轉移性肝癌診斷治療方法,優化綜合治療措施以改善預后,為精準治療復發性轉移性肝癌提供更多證據,推動我國肝癌防治事業的發展。
《肝細胞肝癌全程多學科規范化管理:華西醫院多學科專家共識》編審成員名單
名譽組長:嚴律南
組 長:文天夫
成員(以姓氏拼音為序):
曹丹5 陳恩強6 陳衛霞2 陳哲宇1 黃紀偉1 蔣利1 李波1 李秋5 李志平5 李川1
魯昌立4 盧強3 盧武勝1 羅燕3 劉非1 唐紅6 王文濤1 王辛5 魏永剛1 吳苾2
吳泓1 徐明清1 楊家印1 楊雨5 曾勇1 張鳴1
(1:肝臟外科;2:放射科;3:超聲診斷科;4:病理科;5:腫瘤中心;6:感染性疾病中心)
編寫秘書:張曉赟 彭偉 劉暢 聶世鴻 沈俊頤 金諶
![]() 表1 證據等級
表1 證據等級
| 證據級別 | 描述 |
| Ⅰa | 證據源于對多項隨機對照研究的 Meta 分析結果 |
| Ⅰb | 證據源于至少 1 項設計良好的隨機對照研究結果 |
| Ⅱa | 證據源于至少 1 項設計良好的前瞻性非隨機對照研究結果 |
| Ⅱb | 證據源自至少 1 項設計良好的其他類型干預性臨床研究結果 |
| Ⅲ | 證據源于設計良好的非干預性研究,如描述性研究、相關性研究等 |
| Ⅳ | 證據源于專家委員會報告或權威專家的臨床經驗報道 |
下載CSV
![]() 表2 推薦意見級別
表2 推薦意見級別
| 證據等級 | 描述 |
| A | 良好的科學證據提示該醫療行為帶來明確獲益,建議醫師對患者實施該醫療行為 |
| B | 現有證據表明該醫療行為可帶來中度獲益,超過其潛在風險;醫師可建議對患者實施該醫療行為 |
| C | 現有證據表明該醫療行為可能獲益較小,或獲益與風險接近;醫師可根據患者個體情況有選擇地向患者建議或實施該醫療行為 |
| D | 現有證據表明該醫療行為無獲益,或其潛在風險超過獲益;醫師不宜向患者實施該醫療行為 |
| E | 缺乏科學證據,或現有證據無法評價該醫療行為的獲益與風險;醫師應幫助患者理解該醫療行為存在的不確定性 |
下載CSV
| 1. | Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin, 2015, 65(2): 87-108. |
| 2. | Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin, 2016, 66(2): 115-132. |
| 3. | 中華人民共和國衛計委. 原發性肝癌診療規范 (2017 年版). 中華消化外科雜志, 2017, 16(7): 635-647. |
| 4. | 中華人民共和國衛健委. 原發性肝癌診療規范(2019 年版). 中國實用外科雜志, 2020, 40(2): 121-138. |
| 5. | 四川大學華西醫院肝癌 MDT 團隊. 肝細胞癌切除術后復發轉移的防治: 華西醫院多學科專家共識. 中國普外基礎與臨床雜志, 2017, 24(8): 927-939. |
| 6. | Wen T, Jin C, Facciorusso A, et al. Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: an international expert consensus. Hepatobiliary Surg Nutr, 2018, 7(5): 353-371. |
| 7. | Ryder SD, British Society of gastroenterology. guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut, 2003, 52 Suppl 3(Suppl 3): i1-i8. |
| 8. | Force USPST. Grade definitions and suggestions for practice. 2012. https://www.uspreventiveservicestaskforce.org/Page/Name/grade-definitions. |
| 9. | European Association For The Study Of The Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol, 2012, 56(4): 908-943. |
| 10. | Omata M, Cheng AL, Kokudo N, et al. Asia-pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol Int, 2017, 11(4): 317-370. |
| 11. | Park MJ, Kim YK, Lee MW, et al. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology, 2012, 264(3): 761-770. |
| 12. | Di Martino M, Marin D, Guerrisi A, et al. Intraindividual comparison of gadoxetate disodium-enhanced mr imaging and 64-section multidetector CT in the detection of hepatocellular carcinoma in patients with cirrhosis. Radiology, 2010, 256(3): 806-816. |
| 13. | Mitchell DG, Bruix J, Sherman M, et al. LI-RADS (liver imaging reporting and data system): summary, discussion, and consensus of the LI-RADS management working group and future directions. Hepatology, 2015, 61(3): 1056-1065. |
| 14. | Best J, Bilgi H, Heider D, et al. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z Gastroenterol, 2016, 54(12): 1296-1305. |
| 15. | Johnson PJ. The BALAD-2 and GALAD biomarker models for hepatocellular carcinoma. Gastroenterol Hepatol (N Y), 2017, 13(4): 231-233. |
| 16. | Cong WM, Bu H, Chen J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol, 2016, 22(42): 9279-9287. |
| 17. | Huang C, Zhu XD, Ji Y, et al. Microvascular invasion has limited clinical values in hepatocellular carcinoma patients at Barcelona clinic liver cancer (BCLC) stages 0 or B. BMC Cancer, 2017, 17(1): 58. |
| 18. | 中華醫學會外科學分會肝臟外科學組. 肝細胞癌外科治療方法的選擇專家共識(2016 年第 3 次修訂). 中華消化外科雜志, 2017, 16(2): 113-115. |
| 19. | Shen JY, Li C, Wen TF, et al. Alpha fetoprotein changes predict hepatocellular carcinoma survival beyond the milan criteria after hepatectomy. J Surg Res, 2017, 209: 102-111. |
| 20. | Chen YK, Hsieh DS, Liao CS, et al. Utility of FDG-PET for investigating unexplained serum AFP elevation in patients with suspected hepatocellular carcinoma recurrence. Anticancer Res, 2005, 25(6C): 4719-4725. |
| 21. | 文天夫. 轉化切除是提高肝細胞癌切除率的重要途徑. 中國普外基礎與臨床雜志, 2020, 27(2): 133-136. |
| 22. | Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery, 1990, 107(5): 521-527. |
| 23. | Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg, 2012, 255(3): 405-414. |
| 24. | 周儉, 王征, 孫健, 等. 聯合肝臟離斷和門靜脈結扎的二步肝切除術. 中華消化外科雜志, 2013, 12(7): 485-489. |
| 25. | 彭馳涵, 李川, 文天夫, 等. 原發性肝癌行 ALPPS 的適應證與禁忌證初探 (附 15 例報道). 中國普外基礎與臨床雜志, 2015, 22(10): 1183-1186. |
| 26. | Guiu B, Chevallier P, Denys A, et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol, 2016, 26(12): 4259-4267. |
| 27. | 劉暢, 張曉赟, 金諶, 等. 肝靜脈系統栓堵術在第二階段根治性肝癌切除術中的應用. 中國普外基礎與臨床雜志, 2019, 26(7): 841-846. |
| 28. | Fan J, Tang ZY, Yu YQ, et al. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg, 1998, 15(6): 674-678. |
| 29. | Irtan S, Chopin-Laly X, Ronot M, et al. Complete regression of locally advanced hepatocellular carcinoma induced by sorafenib allowing curative resection. Liver Int, 2011, 31(5): 740-743. |
| 30. | 余業勤, 湯釗猷, 周信達, 等. 大肝癌的分階段治療. 中華外科雜志, 1983, 21(2): 92-93. |
| 31. | Ronot M, Cauchy F, Gregoli B, et al. Sequential transarterial chemoembolization and portal vein embolization before resection is a valid oncological strategy for unilobar hepatocellular carcinoma regardless of the tumor burden. HPB (Oxford), 2016, 18(8): 684-690. |
| 32. | Wang Z, Ren Z, Chen Y, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res, 2018, 24(9): 2074-2081. |
| 33. | Kanai T, Hirohashi S, Upton MP, et al. Pathology of small hepatocellular carcinoma. Cancer, 1987, 60(4): 810-819. |
| 34. | Yang LY, Fang F, Ou DP, et al. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg, 2009, 249(1): 118-123. |
| 35. | Hui AM, Takayama T, Sano K, et al. Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J Hepatol, 2000, 33(6): 975-979. |
| 36. | Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl, 2005, 11(9): 1086-1092. |
| 37. | Shirabe K, Toshima T, Kimura K, et al. New scoring system for prediction of microvascular invasion in patients with hepatocellular carcinoma. Liver Int, 2014, 34(6): 937-941. |
| 38. | Lee S, Kim SH, Lee JE, et al. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol, 2017, 67(3): 526-534. |
| 39. | Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg, 2016, 151(4): 356-363. |
| 40. | Li C, Shen JY, Zhang XY, et al. Predictors of futile liver resection for patients with barcelona clinic liver cancer stage B/C hepatocellular carcinoma. J Gastrointest Surg, 2018, 22(3): 496-502. |
| 41. | Li C, Liu JY, Peng W, et al. Liver resection versus transplantation for multiple hepatocellular carcinoma: a propensity score analysis. Oncotarget, 2017, 8(46): 81492-81500. |
| 42. | 中國醫師協會肝癌專業委員會. 肝細胞癌合并門靜脈癌栓多學科診治中國專家共識 (2018 年版). 中國實用外科雜志, 2019, 39(1): 46-52. |
| 43. | Li J, Yan LN, Yang J, et al. Indicators of prognosis after liver transplantation in chinese hepatocellular carcinoma patients. World J Gastroenterol, 2009, 15(33): 4170-4176. |
| 44. | Yang XR, Xu Y, Yu B, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut, 2010, 59(7): 953-962. |
| 45. | Sun YF, Xu Y, Yang XR, et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology, 2013, 57(4): 1458-1468. |
| 46. | Zhang X, Li J, Shen F, et al. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol, 2018, 33(2): 347-354. |
| 47. | Allard MA, Sebagh M, Ruiz A, et al. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol, 2015, 63(1): 83-92. |
| 48. | Wei X, Jiang Y, Zhang X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol, 2019, 37(24): 2141-2151. |
| 49. | Liu CL, Fan ST, Cheung ST, et al. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg, 2006, 244(2): 194-203. |
| 50. | Zheng JL, Shen S, Jiang L, et al. Outcomes of anterior approach major hepatectomy with diaphragmatic resection for single huge right lobe HCC with diaphragmatic invasion. Medicine (Baltimore), 2018, 97(36): e12194. |
| 51. | Man K, Fan ST, Ng IO, et al. Prospective evaluation of pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg, 1997, 226(6): 704-711. |
| 52. | Wen T, Chen Z, Yan L, et al. Continuous normothermic hemihepatic vascular inflow occlusion over 60 min for hepatectomy in patients with cirrhosis caused by hepatitis B virus. Hepatol Res, 2007, 37(5): 346-352. |
| 53. | Liu L, Wang ZW, Jiang SQ, et al. Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: a meta-analysis. PLoS One, 2013, 8(5): e64261. |
| 54. | Lee KF, Wong J, Cheung SYS, et al. Does intermittent pringle maneuver increase postoperative complications after hepatectomy for hepatocellular carcinoma? A randomized controlled trial. World J Surg, 2018, 42(10): 3302-3311. |
| 55. | Lu Q, Luo Y, Yuan CX, et al. Value of contrast-enhanced intraoperative ultrasound for cirrhotic patients with hepatocellular carcinoma: a report of 20 cases. World J Gastroenterol, 2008, 14(25): 4005-4010. |
| 56. | 方馳華, 張鵬, 羅火靈, 等. 增強現實導航技術聯合吲哚菁綠分子熒光影像在三維腹腔鏡肝切除術中的應用. 中華外科雜志, 2019, 57(8): 578-584. |
| 57. | Wu H, Lu Q, Luo Y, et al. Application of contrast-enhanced intraoperative ultrasonography in the decision-making about hepatocellular carcinoma operation. World J Gastroenterol, 2010, 16(4): 508-512. |
| 58. | Zhou C, Peng Y, Zhou K, et al. Surgical resection plus radiofrequency ablation for the treatment of multifocal hepatocellular carcinoma. Hepatobiliary Surg Nutr, 2019, 8(1): 19-28. |
| 59. | Shindoh J, Makuuchi M, Matsuyama Y, et al. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol, 2016, 64(3): 594-600. |
| 60. | Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg, 2007, 245(1): 36-43. |
| 61. | Shen JY, Li C, Wen TF, et al. A simple prognostic score system predicts the prognosis of solitary large hepatocellular carcinoma following hepatectomy. Medicine (Baltimore), 2016, 95(31): e4296. |
| 62. | Eguchi S, Kanematsu T, Arii S, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery, 2008, 143(4): 469-475. |
| 63. | Han J, Li ZL, Xing H, et al. The impact of resection margin and microvascular invasion on long-term prognosis after curative resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford), 2019, 21(8): 962-971. |
| 64. | Makino Y, Yamanoi A, Kimoto T, et al. The influence of perioperative blood transfusion on intrahepatic recurrence after curative resection of hepatocellular carcinoma. Am J Gastroenterol, 2000, 95(5): 1294-1300. |
| 65. | Buczkowski AK, Kim PT, Ho SG, et al. Multidisciplinary management of ruptured hepatocellular carcinoma. J Gastrointest Surg, 2006, 10(3): 379-386. |
| 66. | Kim BW, Kim YB, Wang HJ, et al. Risk Factors for immediate post-operative fatal recurrence after curative resection of hepatocellular carcinoma. World J Gastroenterol, 2006, 12(1): 99-104. |
| 67. | Zhu WJ, Huang CY, Li C, et al. Risk factors for early recurrence of HBV-related hepatocellular carcinoma meeting milan criteria after curative resection. Asian Pac J Cancer Prev, 2013, 14(12): 7101-7106. |
| 68. | Lee CW, Chan KM, Lee CF, et al. Hepatic resection for hepatocellular carcinoma with lymph node metastasis: clinicopathological analysis and survival outcome. Asian J Surg, 2011, 34(2): 53-62. |
| 69. | Nobuoka D, Kato Y, Gotohda N, et al. Postoperative serum alpha-fetoprotein level is a useful predictor of recurrence after hepatectomy for hepatocellular carcinoma. Oncol Rep, 2010, 24(2): 521-528. |
| 70. | Yin J, Li N, Han Y, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis b virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol, 2013, 31(29): 3647-3655. |
| 71. | 中國臨床腫瘤學會指南工作委員會. 原發性肝癌診療指南. 北京: 人民衛生出版社, 2018: 35-38. |
| 72. | Ren ZG, Lin ZY, Xia JL, et al. Postoperative adjuvant arterial chemoembolization improves survival of hepatocellular carcinoma patients with risk factors for residual tumor: a retrospective control study. World J Gastroenterol, 2004, 10(19): 2791-2794. |
| 73. | Peng BG, He Q, Li JP, et al. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg, 2009, 198(3): 313-318. |
| 74. | Zhong JH, Li LQ. Postoperative adjuvant transarterial chemoembolization for participants with hepatocellular carcinoma: a meta-analysis. Hepatol Res, 2010, 40(10): 943-953. |
| 75. | Li KW, Wen TF, Li X, et al. The effect of postoperative TACE on prognosis of HCC with microscopic venous invasion. Hepatogastroenterology, 2012, 59(118): 1944-1946. |
| 76. | Zhong JH, Li H, Li LQ, et al. Adjuvant therapy options following curative treatment of hepatocellular carcinoma: a systematic review of randomized trials. Eur J Surg Oncol, 2012, 38(4): 286-295. |
| 77. | Wei W, Jian PE, Li SH, et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety. Cancer Commun (Lond), 2018, 38(1): 61. |
| 78. | 程樹群, 吳孟超, 陳漢, 等. 肝癌患者術后肝動脈化療栓塞聯合胸腺肽治療預防復發的隨機對照研究. 中華腫瘤雜志, 2004, 26(5): 305-307. |
| 79. | Gish RG, Gordon SC, Nelson D, et al. A randomized controlled trial of thymalfasin plus transarterial chemoembolization for unresectable hepatocellular carcinoma. Hepatol Int, 2009, 3(3): 480-489. |
| 80. | He C, Peng W, Li C, et al. Thymalfasin, a promising adjuvant therapy in small hepatocellular carcinoma after liver resection. Medicine (Baltimore), 2017, 96(16): e6606. |
| 81. | Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet, 2000, 356(9232): 802-807. |
| 82. | Ikeda K, Arase Y, Saitoh S, et al. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor—A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology, 2000, 32(2): 228-232. |
| 83. | Sun HC, Tang ZY, Wang L, et al. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol, 2006, 132(7): 458-465. |
| 84. | Lo CM, Liu CL, Chan SC, et al. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg, 2007, 245(6): 831-842. |
| 85. | Breitenstein S, Dimitroulis D, Petrowsky H, et al. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg, 2009, 96(9): 975-981. |
| 86. | Wang SN, Chuang SC, Lee KT. Efficacy of sorafenib as adjuvant therapy to prevent early recurrence of hepatocellular carcinoma after curative surgery: a pilot study. Hepatol Res, 2014, 44(5): 523-531. |
| 87. | Zhang W, Zhao G, Wei K, et al. Adjuvant sorafenib reduced mortality and prolonged overall survival and post-recurrence survival in hepatocellular carcinoma patients after curative resection: a single-center experience. Biosci Trends, 2014, 8(6): 333-338. |
| 88. | Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol, 2015, 16(13): 1344-1354. |
| 89. | Kudo M, Finn RS, Qin SK, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet, 2018, 391(10126): 1163-1173. |
| 90. | Hiraoka A, Kumada T, Kariyama K, et al. Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: Multicenter analysis. Cancer Med, 2019, 8(1): 137-146. |
| 91. | Mizuta T, Ozaki I, Eguchi Y, et al. The Effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: a pilot study. Cancer, 2006, 106(4): 867-872. |
| 92. | Kakizaki S, Sohara N, Sato K, et al. Preventive effects of vitamin K on recurrent disease in patients with hepatocellular carcinoma arising from hepatitis C viral infection. J Gastroenterol Hepatol, 2007, 22(4): 518-522. |
| 93. | Yoshida H, Shiratori Y, Kudo M, et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology, 2011, 54(2): 532-540. |
| 94. | Okita K, Izumi N, Matsui O, et al. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: a randomized double-blind placebo-controlled study. J Gastroenterol, 2015, 50(2): 191-202. |
| 95. | Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol, 2013, 31(28): 3501-3508. |
| 96. | Xia Y, Qiu Y, Li J, et al. Adjuvant therapy with capecitabine postpones recurrence of hepatocellular carcinoma after curative resection: a randomized controlled trial. Ann Surg Oncol, 2010, 17(12): 3137-3144. |
| 97. | Sun HC, Zhang W, Qin LX, et al. Positive serum hepatitis B E antigen is associated with higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B-related hepatocellular carcinoma. J Hepatol, 2007, 47(5): 684-690. |
| 98. | Huang G, Lau WY, Wang ZG, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg, 2015, 261(1): 56-66. |
| 99. | Wong JS, Wong GL, Tsoi KK, et al. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther, 2011, 33(10): 1104-1112. |
| 100. | Zhou XD, Tang ZY, Ma ZC, et al. Twenty-year survivors after resection for hepatocellular carcinoma-analysis of 53 cases. J Cancer Res Clin Oncol, 2009, 135(8): 1067-1072. |
| 101. | Si MS, Amersi F, Golish SR, et al. Prevalence of metastases in hepatocellular carcinoma: risk factors and impact on survival. Am Surg, 2003, 69(10): 879-885. |
| 102. | Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol, 2005, 20(11): 1781-1787. |
| 103. | Yang Y, Nagano H, Ota H, et al. Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery, 2007, 141(2): 196-202. |
| 104. | Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer, 2000, 89(3): 500-507. |
| 105. | Lee KF, Chong CCN, Fong AKW, et al. Pattern of disease recurrence and its implications for postoperative surveillance after curative hepatectomy for hepatocellular carcinoma: experience from a single center. Hepatobiliary Surg Nutr, 2018, 7(5): 320-330. |
| 106. | Sakamoto M, Hirohashi S, Tsuda H, et al. Multicentric independent development of hepatocellular carcinoma revealed by analysis of hepatitis B virus integration pattern. Am J Surg Pathol, 1989, 13(12): 1064-1067. |
| 107. | Liver Cancer Study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer. Jpn J Surg, 1989, 19(1): 98-129. |
| 108. | Takenaka K, Adachi E, Nishizaki T, et al. Possible multicentric occurrence of hepatocellular carcinoma: a clinicopathological study. Hepatology, 1994, 19(4): 889-894. |
| 109. | Ng IO, Guan XY, Poon RT, et al. Determination of the molecular relationship between multiple tumour nodules in hepatocellular carcinoma differentiates multicentric origin from intrahepatic metastasis. J Pathol, 2003, 199(3): 345-353. |
| 110. | Chen PJ, Chen DS, Lai MY, et al. Clonal origin of recurrent hepatocellular carcinomas. Gastroenterology, 1989, 96(2 Pt 1): 527-529. |
| 111. | Hodges KB, Cummings OW, Saxena R, et al. Clonal origin of multifocal hepatocellular carcinoma. Cancer, 2010, 116(17): 4078-4085. |
| 112. | Wang B, Xia CY, Lau WY, et al. Determination of clonal origin of recurrent hepatocellular carcinoma for personalized therapy and outcomes evaluation: a new strategy for hepatic surgery. J Am Coll Surg, 2013, 217(6): 1054-1062. |
| 113. | Morimoto O, Nagano H, Sakon M, et al. Diagnosis of intrahepatic metastasis and multicentric carcinogenesis by microsatellite loss of heterozygosity in patients with multiple and recurrent hepatocellular carcinomas. J Hepatol, 2003, 39(2): 215-221. |
| 114. | Esumi M, Aritaka T, Arii M, et al. Clonal origin of human hepatoma determined by integration of hepatitis B virus DNA. Cancer Res, 1986, 46(11): 5767-5771. |
| 115. | Iizuka N, Oka M, Yamada-Okabe H, et al. Oligonucleotide microarray for prediction of early intrahepatic recurrence of hepatocellular carcinoma after curative resection. Lancet, 2003, 361(9361): 923-929. |
| 116. | Cheung ST, Chen X, Guan XY, et al. Identify metastasis-associated genes in hepatocellular carcinoma through clonality delineation for multinodular tumor. Cancer Res, 2002, 62(16): 4711-4721. |
| 117. | Barry CT, D'Souza M, McCall M, et al. Micro RNA expression profiles as adjunctive data to assess the risk of hepatocellular carcinoma recurrence after liver transplantation. Am J Transplant, 2012, 12(2): 428-437. |
| 118. | Wilkens L, Bredt M, Flemming P, et al. Differentiation of multicentric origin from intra-organ metastatic spread of hepatocellular carcinomas by comparative genomic hybridization. J Pathol, 2000, 192(1): 43-51. |
| 119. | Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med, 2012, 366(10): 883-892. |
| 120. | Miao R, Luo H, Zhou H, et al. Identification of prognostic biomarkers in hepatitis B virus-related hepatocellular carcinoma and stratification by integrative multi-omics analysis. J Hepatol, 2014, 61(4): 840-849. |
| 121. | Xu XF, Xing H, Han J, et al. Risk Factors, Patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg, 2019, 154(3): 209-217. |
| 122. | Xing H, Zhang WG, Cescon M, et al. Defining and predicting early recurrence after liver resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford), 2020, 22(5): 677-689. |
| 123. | Erridge S, Pucher PH, Markar SR, et al. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg, 2017, 104(11): 1433-1442. |
| 124. | Meniconi RL, Komatsu S, Perdigao F, et al. Recurrent hepatocellular carcinoma: a western strategy that emphasizes the impact of pathologic profile of the first resection. Surgery, 2015, 157(3): 454-462. |
| 125. | Zhang X, Li C, Wen T, et al. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol, 2015, 27(8): 933-940. |
| 126. | Wang DY, Liu L, Qi XS, et al. Hepatic re-resection versus transarterial chemoembolization for the treatment of recurrent hepatocellular carcinoma after initial resection: a systematic review and meta-analysis. Asian Pac J Cancer Prev, 2015, 16(13): 5573-5578. |
| 127. | Mise Y, Hasegawa K, Shindoh J, et al. The feasibility of third or more repeat hepatectomy for recurrent hepatocellular carcinoma. Ann Surg, 2015, 262(2): 347-357. |
| 128. | Yamashita Y, Shirabe K, Tsuijita E, et al. Third or more repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery, 2013, 154(5): 1038-1045. |
| 129. | Wu CC, Cheng SB, Yeh DC, et al. Second and third hepatectomies for recurrent hepatocellular carcinoma are justified. Br J Surg, 2009, 96(9): 1049-1057. |
| 130. | Poon RT, Fan ST, O'Suilleabhain CB, et al. Aggressive management of patients with extrahepatic and intrahepatic recurrences of hepatocellular carcinoma by combined resection and locoregional therapy. J Am Coll Surg, 2002, 195(3): 311-318. |
| 131. | Minagawa M, Makuuchi M, Takayama T, et al. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg, 2003, 238(5): 703-710. |
| 132. | Zou Q, Li J, Wu D, et al. Nomograms for pre-operative and post-operative prediction of long-term survival of patients who underwent repeat hepatectomy for recurrent hepatocellular carcinoma. Ann Surg Oncol, 2016, 23(8): 2618-2626. |
| 133. | Yamashita S, Aoki T, Inoue Y, et al. Outcome of salvage hepatic resection for recurrent hepatocellular carcinoma after radiofrequency ablation therapy. Surgery, 2015, 157(3): 463-472. |
| 134. | Nakajima Y, Ko S, Kanamura T, et al. Repeat liver resection for hepatocellular carcinoma. J Am Coll Surg, 2001, 192(3): 339-344. |
| 135. | 董家鴻, 鄭樹森, 陳孝平, 等. 肝切除術前肝臟儲備功能評估的專家共識(2011 版). 中華消化外科雜志, 2011, 10(1): 20-25. |
| 136. | Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology, 1997, 26(5): 1176-1181. |
| 137. | Shirabe K, Shimada M, Gion T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg, 1999, 188(3): 304-309. |
| 138. | Shindoh J, D Tzeng CW, Vauthey JN. Portal vein embolization for hepatocellular carcinoma. Liver Cancer, 2012, 1(3-4): 159-167. |
| 139. | Siriwardana RC, Lo CM, Chan SC, et al. Role of portal vein embolization in hepatocellular carcinoma management and its effect on recurrence: a case-control study. World J Surg, 2012, 36(7): 1640-1646. |
| 140. | Tsujita E, Utsunomiya T, Ohta M, et al. Outcome of repeat hepatectomy in patients with hepatocellular carcinoma aged 75 years and older. Surgery, 2010, 147(5): 696-703. |
| 141. | Liu K, Chen Y, Wu X, et al. Laparoscopic liver re-resection is feasible for patients with posthepatectomy hepatocellular carcinoma recurrence: a propensity score matching study. Surg Endosc, 2017, 31(11): 4790-4798. |
| 142. | Chan AC, Poon RT, Chok KS, et al. Feasibility of laparoscopic re-resection for patients with recurrent hepatocellular carcinoma. World J Surg, 2014, 38(5): 1141-1146. |
| 143. | Hu M, Zhao G, Xu D, et al. Laparoscopic repeat resection of recurrent hepatocellular carcinoma. World J Surg, 2011, 35(3): 648-655. |
| 144. | Zhang J, Zhou ZG, Huang ZX, et al. Prospective, single-center cohort study analyzing the efficacy of complete laparoscopic resection on recurrent hepatocellular carcinoma. Chin J Cancer, 2016, 35: 25. |
| 145. | Kanazawa A, Tsukamoto T, Shimizu S, et al. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Sci, 2013, 20(5): 512-517. |
| 146. | Belli G, Cioffi L, Fantini C, et al. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: feasibility, safety, and results. Surg Endosc, 2009, 23(8): 1807-1811. |
| 147. | Halls MC, Cipriani F, Berardi G, et al. Conversion for unfavorable intraoperative events results in significantly worse outcomes during laparoscopic liver resection: lessons learned from a multicenter review of 2861 cases. Ann Surg, 2018, 268(6): 1051-1057. |
| 148. | Fan J, Wu ZQ, Zhou J, et al. Hepatocellular carcinoma associated with tumor thrombosis in the portal vein: the effects of different treatments. Hepatobiliary Pancreat Dis Int, 2003, 2(4): 513-519. |
| 149. | Ye JZ, Wang YY, Bai T, et al. Surgical resection for hepatocellular carcinoma with portal vein tumor thrombus in the asia-pacific region beyond the barcelona clinic liver cancer treatment algorithms: a review and update. Oncotarget, 2017, 8(54): 93258-93278. |
| 150. | Tantawi B, Cherqui D, Tran van Nhieu J, et al. Surgery for biliary obstruction by tumour thrombus in primary liver cancer. Br J Surg, 1996, 83(11): 1522-1525. |
| 151. | Qin LX, Ma ZC, Wu ZQ, et al. Diagnosis and surgical treatments of hepatocellular carcinoma with tumor thrombosis in bile duct: experience of 34 patients. World J Gastroenterol, 2004, 10(10): 1397-1401. |
| 152. | Satoh S, Ikai I, Honda G, et al. Clinicopathologic evaluation of hepatocellular carcinoma with bile duct thrombi. Surgery, 2000, 128(5): 779-783. |
| 153. | Liu QY, Lai DM, Liu C, et al. A Special recurrent pattern in small hepatocellular carcinoma after treatment: bile duct tumor thrombus formation. World J Gastroenterol, 2011, 17(43): 4817-4824. |
| 154. | Hanaoka J, Shimada M, Ikegami T, et al. Hepatocellular carcinoma with massive bile duct tumor thrombus: report of a long-term survival. Hepatogastroenterology, 2008, 55(88): 2217-2220. |
| 155. | Moon DB, Hwang S, Wang HJ, et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: a Korean multicenter study. World J Surg, 2013, 37(2): 443-451. |
| 156. | Wong TC, Cheung TT, Chok KS, et al. Outcomes of hepatectomy for hepatocellular carcinoma with bile duct tumour thrombus. HPB (Oxford), 2015, 17(5): 401-408. |
| 157. | Xu LB, Liu C. Hepatocellular carcinoma with bile duct tumor thrombus: extrahepatic bile duct preserving or not? Ann Surg, 2017, 266(6): e62-e63. |
| 158. | Chen XP, Wu ZD, Huang ZY, et al. Use of hepatectomy and splenectomy to treat hepatocellular carcinoma with cirrhotic hypersplenism. Br J Surg, 2005, 92(3): 334-339. |
| 159. | Zhang X, Li C, Wen T, et al. Synchronous splenectomy and hepatectomy for patients with small hepatocellular carcinoma and pathological spleen: neutrophil to lymphocyte ratio changes can predict the prognosis. Oncotarget, 2017, 8(28): 46298-46311. |
| 160. | Zhang XY, Li C, Wen TF, et al. Synchronous splenectomy and hepatectomy for patients with hepatocellular carcinoma and hypersplenism: a case-control study. World J Gastroenterol, 2015, 21(8): 2358-2366. |
| 161. | Zhang Y, Wen TF, Yan LN, et al. Preoperative predictors of portal vein thrombosis after splenectomy with periesophagogastric devascularization. World J Gastroenterol, 2012, 18(15): 1834-1839. |
| 162. | 張曉赟, 李川, 彭偉, 等. 血小板/脾臟體積比預測肝硬化門靜脈高壓癥的嚴重程度. 腹部外科, 2016, 29(3): 187-192. |
| 163. | 張宇, 文天夫, 陳哲宇, 等. 術前門靜脈血流速度在門靜脈高壓癥斷流術后血栓形成中的預測價值. 中華外科雜志, 2009, 47(11): 825-828. |
| 164. | Lu MD, Yin XY, Xie XY, et al. Percutaneous thermal ablation for recurrent hepatocellular carcinoma after hepatectomy. Br J Surg, 2005, 92(11): 1393-1398. |
| 165. | Thomasset SC, Dennison AR, Garcea G. Ablation for recurrent hepatocellular carcinoma: a systematic review of clinical efficacy and prognostic factors. World J Surg, 2015, 39(5): 1150-1160. |
| 166. | Chen HW, Lai EC, Zhen ZJ, et al. Ultrasound-guided percutaneous cryotherapy of hepatocellular carcinoma. Int J Surg, 2011, 9(2): 188-191. |
| 167. | Chen R, Gan Y, Ge N, et al. Transarterial chemoembolization versus radiofrequency ablation for recurrent hepatocellular carcinoma after resection within barcelona clinic liver cancer stage 0/a: a retrospective comparative study. J Vasc Interv Radiol, 2016, 27(12): 1829-1836. |
| 168. | Wang K, Liu G, Li J, et al. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol, 2015, 41(2): 236-242. |
| 169. | 段紀成, 劉凱, 吳孟超, 等. 經皮肝穿刺射頻消融與再次肝切除對復發性小肝癌的隨機對照研究. 肝膽外科雜志, 2015, 23(1): 15-17. |
| 170. | Chan AC, Poon RT, Cheung TT, et al. Survival analysis of re-resection versus radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. World J Surg, 2012, 36(1): 151-156. |
| 171. | Ho CM, Lee PH, Shau WY, et al. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery, 2012, 151(5): 700-709. |
| 172. | Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg, 2006, 243(3): 321-328. |
| 173. | Huang JW, Yan LN, Cheng ZY, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg, 2010, 252(6): 903-912. |
| 174. | 中國抗癌協會肝癌專業委員會, 中國抗癌協會臨床腫瘤學協作專業委員會, 中華醫學會肝病學分會肝癌學組. 肝癌局部消融治療規范的專家共識. 實用肝臟病雜志, 2011, 14(4): 243-245. |
| 175. | Liang HH, Chen MS, Peng ZW, et al. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol, 2008, 15(12): 3484-3493. |
| 176. | Chen MH, Yang W, Yan K, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients—mathematic model, overlapping mode, and electrode placement process. Radiology, 2004, 232(1): 260-271. |
| 177. | Zhang L, Wang N, Shen Q, et al. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS One, 2013, 8(10): e76119. |
| 178. | Abdelaziz A, Elbaz T, Shousha HI, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an egyptian multidisciplinary clinic experience. Surg Endosc, 2014, 28(12): 3429-3434. |
| 179. | Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology, 2012, 262(1): 43-58. |
| 180. | Farina L, Weiss N, Nissenbaum Y, et al. Characterisation of tissue shrinkage during microwave thermal ablation. Int J Hyperthermia, 2014, 30(7): 419-428. |
| 181. | Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia, 2016, 32(3): 339-344. |
| 182. | Luo W, Zhang Y, He G, et al. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol, 2017, 15(1): 126. |
| 183. | Ei S, Hibi T, Tanabe M, et al. Cryoablation provides superior local control of primary hepatocellular carcinomas of >2 cm compared with radiofrequency ablation and microwave coagulation therapy: an underestimated tool in the toolbox. Ann Surg Oncol, 2015, 22(4): 1294-1300. |
| 184. | Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology, 2015, 61(5): 1579-1590. |
| 185. | Chan AC, Cheung TT, Fan ST, et al. Survival analysis of high-intensity focused ultrasound therapy versus radiofrequency ablation in the treatment of recurrent hepatocellular carcinoma. Ann Surg, 2013, 257(4): 686-692. |
| 186. | Giorgio A, Di Sarno A, De Stefano G, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Res, 2011, 31(6): 2291-2295. |
| 187. | Brunello F, Veltri A, Carucci P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand J Gastroenterol, 2008, 43(6): 727-735. |
| 188. | Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology, 2005, 129(1): 122-130. |
| 189. | Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology, 2003, 228(1): 235-240. |
| 190. | Yong CC, Tsai MC, Lin CC, et al. Comparison of salvage living donor liver transplantation and local regional therapy for recurrent hepatocellular carcinoma. World J Surg, 2016, 40(10): 2472-2480. |
| 191. | Chan AC, Chan SC, Chok KS, et al. Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl, 2013, 19(4): 411-419. |
| 192. | Yamashita Y, Yoshida Y, Kurihara T, et al. Surgical results for recurrent hepatocellular carcinoma after curative hepatectomy: repeat hepatectomy versus salvage living donor liver transplantation. Liver Transpl, 2015, 21(7): 961-968. |
| 193. | Del Gaudio M, Ercolani G, Ravaioli M, et al. Liver transplantation for recurrent hepatocellular carcinoma on cirrhosis after liver resection: university of bologna experience. Am J Transplant, 2008, 8(6): 1177-1185. |
| 194. | Lee HS, Choi GH, Joo DJ, et al. The clinical behavior of transplantable recurrent hepatocellular carcinoma after curative resection: implications for salvage liver transplantation. Ann Surg Oncol, 2014, 21(8): 2717-2724. |
| 195. | Majno PE, Sarasin FP, Mentha G, et al. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology, 2000, 31(4): 899-906. |
| 196. | Hu Z, Zhou J, Li Z, et al. Time interval to recurrence as a predictor of overall survival in salvage liver transplantation for patients with hepatocellular carcinoma associated with hepatitis B virus. Surgery, 2015, 157(2): 239-248. |
| 197. | Guerrini GP, Gerunda GE, Montalti R, et al. Results of salvage liver transplantation. Liver Int, 2014, 34(6): e96-e104. |
| 198. | Hu RH, Ho MC, Wu YM, et al. Feasibility of salvage liver transplantation for patients with recurrent hepatocellular carcinoma. Clin Transplant, 2005, 19(2): 175-180. |
| 199. | Kaido T, Mori A, Ogura Y, et al. Living donor liver transplantation for recurrent hepatocellular carcinoma after liver resection. Surgery, 2012, 151(1): 55-60. |
| 200. | Sala M, Fuster J, Llovet JM, et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl, 2004, 10(10): 1294-1300. |
| 201. | Ferrer-Fàbrega J, Forner A, Liccioni A, et al. Prospective validation of Ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology, 2016, 63(3): 839-849. |
| 202. | Yang PC, Ho CM, Hu RH, et al. Prophylactic liver transplantation for high-risk recurrent hepatocellular carcinoma. World J Hepatol, 2016, 8(31): 1309-1317. |
| 203. | Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology, 2002, 35(5): 1164-1171. |
| 204. | Llovet JM, Real MI, Monta?a X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet, 2002, 359(9319): 1734-1739. |
| 205. | Jin YJ, Chung YH, Kim JA, et al. Predisposing factors of hepatocellular carcinoma recurrence following complete remission in response to transarterial chemoembolization. Dig Dis Sci, 2013, 58(6): 1758-1765. |
| 206. | Wang Q, Xia D, Bai W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: a multicentre observational study. J Hepatol, 2019, 70(5): 893-903. |
| 207. | Koh PS, Chan AC, Cheung TT, et al. Efficacy of radiofrequency ablation compared with transarterial chemoembolization for the treatment of recurrent hepatocellular carcinoma: a comparative survival analysis. HPB (Oxford), 2016, 18(1): 72-78. |
| 208. | Cheng YC, Chen TW, Fan HL, et al. Transarterial chemo-embolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant, 2014, 19: 309-316. |
| 209. | Jin YJ, Lee JW, Lee OH, et al. Transarterial chemoembolization versus surgery/radiofrequency ablation for recurrent hepatocellular carcinoma with or without microvascular invasion. J Gastroenterol Hepatol, 2014, 29(5): 1056-1064. |
| 210. | Yang W, Chen MH, Wang MQ, et al. Combination therapy of radiofrequency ablation and transarterial chemoembolization in recurrent hepatocellular carcinoma after hepatectomy compared with single treatment. Hepatol Res, 2009, 39(3): 231-240. |
| 211. | Dai WC, Cheung TT. Strategic overview on the best treatment option for intrahepaitc hepatocellular carcinoma recurrence. Expert Rev Anticancer Ther, 2016, 16(10): 1063-1072. |
| 212. | 曾昭沖. 消融治療后復發肝癌及肝外轉移的放射治療. 中華醫學雜志, 2015, 95(27): 2164-2166. |
| 213. | Bae SH, Park HC, Lim DH, et al. Salvage treatment with hypofractionated radiotherapy in patients with recurrent small hepatocellular carcinoma. Int J Radiat Oncol Biol Phys, 2012, 82(4): e603-607. |
| 214. | Su TS, Liang P, Lu HZ, et al. Stereotactic body radiation therapy for small primary or recurrent hepatocellular carcinoma in 132 Chinese patients. J Surg Oncol, 2016, 113(2): 181-187. |
| 215. | Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol, 2014, 53(3): 399-404. |
| 216. | Seo YS, Kim MS, Yoo HJ, et al. Radiofrequency ablation versus stereotactic body radiotherapy for small hepatocellular carcinoma: a markov model-based analysis. Cancer Med, 2016, 5(11): 3094-3101. |
| 217. | Nakazawa T, Adachi S, Kitano M, et al. Potential prognostic benefits of radiotherapy as an initial treatment for patients with unresectable advanced hepatocellular carcinoma with invasion to intrahepatic large vessels. Oncology, 2007, 73(1-2): 90-97. |
| 218. | Otsuka M, Ohara K, Takada Y, et al. Radiation therapy for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. Int J Clin Oncol, 2003, 8(3): 151-155. |
| 219. | Huang WY, Jen YM, Lee MS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys, 2012, 84(2): 355-361. |
| 220. | He J, Zeng ZC, Tang ZY, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer, 2009, 115(12): 2710-2720. |
| 221. | Chang UK, Kim MS, Han CJ, et al. Clinical result of stereotactic radiosurgery for spinal metastasis from hepatocellular carcinoma: comparison with conventional radiation therapy. J Neurooncol, 2014, 119(1): 141-148. |
| 222. | Byun HK, Kim HJ, Im YR, et al. Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma. Radiother Oncol, 2019, 133: 1-8. |
| 223. | 中華醫學會放射腫瘤學分會, 中國生物醫學工程學會精確放療分會, 肝癌學組與消化系統腫瘤專家委員會, 等. 2016 年原發性肝癌放療共識. 中華放射腫瘤學雜志, 2016, 25(11): 1141-1150. |
| 224. | Rivera L, Giap H, Miller W, et al. Hepatic intra-arterial infusion of yttrium-90 microspheres in the treatment of recurrent hepatocellular carcinoma after liver transplantation: a case report. World J Gastroenterol, 2006, 12(35): 5729-5732. |
| 225. | 中華醫學會核醫學分會轉移性骨腫瘤治療工作委員會. 氯化鍶[89Sr]治療轉移性骨腫瘤專家共識(2017 年版). 中華核醫學與分子影像雜志, 2018, 38(6): 412-415. |
| 226. | Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology, 2016, 151(6): 1155-1163. |
| 227. | Peng ZW, Zhang YJ, Liang HH, et al. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and rf ablation versus rf ablation alone: a prospective randomized trial. Radiology, 2012, 262(2): 689-700. |
| 228. | Khan MA, Combs CS, Brunt EM, et al. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol, 2000, 32(5): 792-797. |
| 229. | Ali SA, Amin DH, Abdelkhalek YI. Efficiency of whole-body 18F-FDG PET CT in detecting the cause of rising serum AFP level in post-therapeutic follow-up for HCC patients. Jpn J Radiol, 2020, 38(5): 472-479. |
| 230. | Nakano M, Tanaka M, Kuromatsu R, et al. Sorafenib for the treatment of advanced hepatocellular carcinoma with extrahepatic metastasis: a prospective multicenter cohort study. Cancer Med, 2015, 4(12): 1836-1843. |
| 231. | Sohn W, Paik YH, Cho JY, et al. Sorafenib therapy for hepatocellular carcinoma with extrahepatic spread: treatment outcome and prognostic factors. J Hepatol, 2015, 62(5): 1112-1121. |
| 232. | Zhang SM, Zeng ZC, Tang ZY, et al. Prognostic analysis of pulmonary metastases from hepatocellular carcinoma. Hepatol Int, 2008, 2(2): 237-243. |
| 233. | Yoon YS, Kim HK, Kim J, et al. Long-term survival and prognostic factors after pulmonary metastasectomy in hepatocellular carcinoma. Ann Surg Oncol, 2010, 17(10): 2795-2801. |
| 234. | Chen F, Sato K, Fujinaga T, et al. Pulmonary resection for metastases from hepatocellular carcinoma. World J Surg, 2008, 32(10): 2213-2217. |
| 235. | Kobayashi S, Takahashi S, Kato Y, et al. Surgical treatment of lymph node metastases from hepatocellular carcinoma. J Hepatobiliary Pancreat Sci, 2011, 18(4): 559-566. |
| 236. | Ha TY, Hwang S, Ahn CS, et al. Resection of metachronous adrenal metastasis after liver resection and transplantation for hepatocellular carcinoma. Dig Surg, 2014, 31(6): 428-435. |
| 237. | Kim SU, Kim DY, Park JY, et al. Hepatocellular carcinoma presenting with bone metastasis: clinical characteristics and prognostic factors. J Cancer Res Clin Oncol, 2008, 134(12): 1377-1384. |
| 238. | Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver Int, 2005, 25(2): 261-265. |
| 239. | Xu L, Wang J, Kim Y, et al. A randomized controlled trial on patients with or without adjuvant autologous cytokine-induced killer cells after curative resection for hepatocellular carcinoma. Oncoimmunology, 2015, 5(3): e1083671. |
| 240. | Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase Ⅲ trial. J Clin Oncol, 2020, 38(3): 193-202. |
| 241. | Sangro B, Park JW, Cruz D, et al. A randomized, multicenter, phase 3 study of nivolumab vs sorafenib as first-line treatment in patients (pts) with advanced hepatocellular carcinoma (HCC): CheckMate-459. J Clin Oncol, 2016, 34(15 Suppl): TPS4147. |
| 242. | Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med, 2020, 382(20): 1894-1905. |
| 243. | Qin SK, Ren ZG, Meng ZQ, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol, 2020, 21(4): 571-580. |
| 244. | Chen Q, Shu C, Laurence AD, et al. Effect of huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut, 2018, 67(11): 2006-2016. |
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin, 2015, 65(2): 87-108.
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin, 2016, 66(2): 115-132.
- 3. 中華人民共和國衛計委. 原發性肝癌診療規范 (2017 年版). 中華消化外科雜志, 2017, 16(7): 635-647.
- 4. 中華人民共和國衛健委. 原發性肝癌診療規范(2019 年版). 中國實用外科雜志, 2020, 40(2): 121-138.
- 5. 四川大學華西醫院肝癌 MDT 團隊. 肝細胞癌切除術后復發轉移的防治: 華西醫院多學科專家共識. 中國普外基礎與臨床雜志, 2017, 24(8): 927-939.
- 6. Wen T, Jin C, Facciorusso A, et al. Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: an international expert consensus. Hepatobiliary Surg Nutr, 2018, 7(5): 353-371.
- 7. Ryder SD, British Society of gastroenterology. guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut, 2003, 52 Suppl 3(Suppl 3): i1-i8.
- 8. Force USPST. Grade definitions and suggestions for practice. 2012. https://www.uspreventiveservicestaskforce.org/Page/Name/grade-definitions.
- 9. European Association For The Study Of The Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol, 2012, 56(4): 908-943.
- 10. Omata M, Cheng AL, Kokudo N, et al. Asia-pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol Int, 2017, 11(4): 317-370.
- 11. Park MJ, Kim YK, Lee MW, et al. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology, 2012, 264(3): 761-770.
- 12. Di Martino M, Marin D, Guerrisi A, et al. Intraindividual comparison of gadoxetate disodium-enhanced mr imaging and 64-section multidetector CT in the detection of hepatocellular carcinoma in patients with cirrhosis. Radiology, 2010, 256(3): 806-816.
- 13. Mitchell DG, Bruix J, Sherman M, et al. LI-RADS (liver imaging reporting and data system): summary, discussion, and consensus of the LI-RADS management working group and future directions. Hepatology, 2015, 61(3): 1056-1065.
- 14. Best J, Bilgi H, Heider D, et al. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z Gastroenterol, 2016, 54(12): 1296-1305.
- 15. Johnson PJ. The BALAD-2 and GALAD biomarker models for hepatocellular carcinoma. Gastroenterol Hepatol (N Y), 2017, 13(4): 231-233.
- 16. Cong WM, Bu H, Chen J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol, 2016, 22(42): 9279-9287.
- 17. Huang C, Zhu XD, Ji Y, et al. Microvascular invasion has limited clinical values in hepatocellular carcinoma patients at Barcelona clinic liver cancer (BCLC) stages 0 or B. BMC Cancer, 2017, 17(1): 58.
- 18. 中華醫學會外科學分會肝臟外科學組. 肝細胞癌外科治療方法的選擇專家共識(2016 年第 3 次修訂). 中華消化外科雜志, 2017, 16(2): 113-115.
- 19. Shen JY, Li C, Wen TF, et al. Alpha fetoprotein changes predict hepatocellular carcinoma survival beyond the milan criteria after hepatectomy. J Surg Res, 2017, 209: 102-111.
- 20. Chen YK, Hsieh DS, Liao CS, et al. Utility of FDG-PET for investigating unexplained serum AFP elevation in patients with suspected hepatocellular carcinoma recurrence. Anticancer Res, 2005, 25(6C): 4719-4725.
- 21. 文天夫. 轉化切除是提高肝細胞癌切除率的重要途徑. 中國普外基礎與臨床雜志, 2020, 27(2): 133-136.
- 22. Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery, 1990, 107(5): 521-527.
- 23. Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg, 2012, 255(3): 405-414.
- 24. 周儉, 王征, 孫健, 等. 聯合肝臟離斷和門靜脈結扎的二步肝切除術. 中華消化外科雜志, 2013, 12(7): 485-489.
- 25. 彭馳涵, 李川, 文天夫, 等. 原發性肝癌行 ALPPS 的適應證與禁忌證初探 (附 15 例報道). 中國普外基礎與臨床雜志, 2015, 22(10): 1183-1186.
- 26. Guiu B, Chevallier P, Denys A, et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol, 2016, 26(12): 4259-4267.
- 27. 劉暢, 張曉赟, 金諶, 等. 肝靜脈系統栓堵術在第二階段根治性肝癌切除術中的應用. 中國普外基礎與臨床雜志, 2019, 26(7): 841-846.
- 28. Fan J, Tang ZY, Yu YQ, et al. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg, 1998, 15(6): 674-678.
- 29. Irtan S, Chopin-Laly X, Ronot M, et al. Complete regression of locally advanced hepatocellular carcinoma induced by sorafenib allowing curative resection. Liver Int, 2011, 31(5): 740-743.
- 30. 余業勤, 湯釗猷, 周信達, 等. 大肝癌的分階段治療. 中華外科雜志, 1983, 21(2): 92-93.
- 31. Ronot M, Cauchy F, Gregoli B, et al. Sequential transarterial chemoembolization and portal vein embolization before resection is a valid oncological strategy for unilobar hepatocellular carcinoma regardless of the tumor burden. HPB (Oxford), 2016, 18(8): 684-690.
- 32. Wang Z, Ren Z, Chen Y, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res, 2018, 24(9): 2074-2081.
- 33. Kanai T, Hirohashi S, Upton MP, et al. Pathology of small hepatocellular carcinoma. Cancer, 1987, 60(4): 810-819.
- 34. Yang LY, Fang F, Ou DP, et al. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg, 2009, 249(1): 118-123.
- 35. Hui AM, Takayama T, Sano K, et al. Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J Hepatol, 2000, 33(6): 975-979.
- 36. Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl, 2005, 11(9): 1086-1092.
- 37. Shirabe K, Toshima T, Kimura K, et al. New scoring system for prediction of microvascular invasion in patients with hepatocellular carcinoma. Liver Int, 2014, 34(6): 937-941.
- 38. Lee S, Kim SH, Lee JE, et al. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol, 2017, 67(3): 526-534.
- 39. Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg, 2016, 151(4): 356-363.
- 40. Li C, Shen JY, Zhang XY, et al. Predictors of futile liver resection for patients with barcelona clinic liver cancer stage B/C hepatocellular carcinoma. J Gastrointest Surg, 2018, 22(3): 496-502.
- 41. Li C, Liu JY, Peng W, et al. Liver resection versus transplantation for multiple hepatocellular carcinoma: a propensity score analysis. Oncotarget, 2017, 8(46): 81492-81500.
- 42. 中國醫師協會肝癌專業委員會. 肝細胞癌合并門靜脈癌栓多學科診治中國專家共識 (2018 年版). 中國實用外科雜志, 2019, 39(1): 46-52.
- 43. Li J, Yan LN, Yang J, et al. Indicators of prognosis after liver transplantation in chinese hepatocellular carcinoma patients. World J Gastroenterol, 2009, 15(33): 4170-4176.
- 44. Yang XR, Xu Y, Yu B, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut, 2010, 59(7): 953-962.
- 45. Sun YF, Xu Y, Yang XR, et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology, 2013, 57(4): 1458-1468.
- 46. Zhang X, Li J, Shen F, et al. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol, 2018, 33(2): 347-354.
- 47. Allard MA, Sebagh M, Ruiz A, et al. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol, 2015, 63(1): 83-92.
- 48. Wei X, Jiang Y, Zhang X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol, 2019, 37(24): 2141-2151.
- 49. Liu CL, Fan ST, Cheung ST, et al. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg, 2006, 244(2): 194-203.
- 50. Zheng JL, Shen S, Jiang L, et al. Outcomes of anterior approach major hepatectomy with diaphragmatic resection for single huge right lobe HCC with diaphragmatic invasion. Medicine (Baltimore), 2018, 97(36): e12194.
- 51. Man K, Fan ST, Ng IO, et al. Prospective evaluation of pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg, 1997, 226(6): 704-711.
- 52. Wen T, Chen Z, Yan L, et al. Continuous normothermic hemihepatic vascular inflow occlusion over 60 min for hepatectomy in patients with cirrhosis caused by hepatitis B virus. Hepatol Res, 2007, 37(5): 346-352.
- 53. Liu L, Wang ZW, Jiang SQ, et al. Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: a meta-analysis. PLoS One, 2013, 8(5): e64261.
- 54. Lee KF, Wong J, Cheung SYS, et al. Does intermittent pringle maneuver increase postoperative complications after hepatectomy for hepatocellular carcinoma? A randomized controlled trial. World J Surg, 2018, 42(10): 3302-3311.
- 55. Lu Q, Luo Y, Yuan CX, et al. Value of contrast-enhanced intraoperative ultrasound for cirrhotic patients with hepatocellular carcinoma: a report of 20 cases. World J Gastroenterol, 2008, 14(25): 4005-4010.
- 56. 方馳華, 張鵬, 羅火靈, 等. 增強現實導航技術聯合吲哚菁綠分子熒光影像在三維腹腔鏡肝切除術中的應用. 中華外科雜志, 2019, 57(8): 578-584.
- 57. Wu H, Lu Q, Luo Y, et al. Application of contrast-enhanced intraoperative ultrasonography in the decision-making about hepatocellular carcinoma operation. World J Gastroenterol, 2010, 16(4): 508-512.
- 58. Zhou C, Peng Y, Zhou K, et al. Surgical resection plus radiofrequency ablation for the treatment of multifocal hepatocellular carcinoma. Hepatobiliary Surg Nutr, 2019, 8(1): 19-28.
- 59. Shindoh J, Makuuchi M, Matsuyama Y, et al. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol, 2016, 64(3): 594-600.
- 60. Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg, 2007, 245(1): 36-43.
- 61. Shen JY, Li C, Wen TF, et al. A simple prognostic score system predicts the prognosis of solitary large hepatocellular carcinoma following hepatectomy. Medicine (Baltimore), 2016, 95(31): e4296.
- 62. Eguchi S, Kanematsu T, Arii S, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery, 2008, 143(4): 469-475.
- 63. Han J, Li ZL, Xing H, et al. The impact of resection margin and microvascular invasion on long-term prognosis after curative resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford), 2019, 21(8): 962-971.
- 64. Makino Y, Yamanoi A, Kimoto T, et al. The influence of perioperative blood transfusion on intrahepatic recurrence after curative resection of hepatocellular carcinoma. Am J Gastroenterol, 2000, 95(5): 1294-1300.
- 65. Buczkowski AK, Kim PT, Ho SG, et al. Multidisciplinary management of ruptured hepatocellular carcinoma. J Gastrointest Surg, 2006, 10(3): 379-386.
- 66. Kim BW, Kim YB, Wang HJ, et al. Risk Factors for immediate post-operative fatal recurrence after curative resection of hepatocellular carcinoma. World J Gastroenterol, 2006, 12(1): 99-104.
- 67. Zhu WJ, Huang CY, Li C, et al. Risk factors for early recurrence of HBV-related hepatocellular carcinoma meeting milan criteria after curative resection. Asian Pac J Cancer Prev, 2013, 14(12): 7101-7106.
- 68. Lee CW, Chan KM, Lee CF, et al. Hepatic resection for hepatocellular carcinoma with lymph node metastasis: clinicopathological analysis and survival outcome. Asian J Surg, 2011, 34(2): 53-62.
- 69. Nobuoka D, Kato Y, Gotohda N, et al. Postoperative serum alpha-fetoprotein level is a useful predictor of recurrence after hepatectomy for hepatocellular carcinoma. Oncol Rep, 2010, 24(2): 521-528.
- 70. Yin J, Li N, Han Y, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis b virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol, 2013, 31(29): 3647-3655.
- 71. 中國臨床腫瘤學會指南工作委員會. 原發性肝癌診療指南. 北京: 人民衛生出版社, 2018: 35-38.
- 72. Ren ZG, Lin ZY, Xia JL, et al. Postoperative adjuvant arterial chemoembolization improves survival of hepatocellular carcinoma patients with risk factors for residual tumor: a retrospective control study. World J Gastroenterol, 2004, 10(19): 2791-2794.
- 73. Peng BG, He Q, Li JP, et al. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg, 2009, 198(3): 313-318.
- 74. Zhong JH, Li LQ. Postoperative adjuvant transarterial chemoembolization for participants with hepatocellular carcinoma: a meta-analysis. Hepatol Res, 2010, 40(10): 943-953.
- 75. Li KW, Wen TF, Li X, et al. The effect of postoperative TACE on prognosis of HCC with microscopic venous invasion. Hepatogastroenterology, 2012, 59(118): 1944-1946.
- 76. Zhong JH, Li H, Li LQ, et al. Adjuvant therapy options following curative treatment of hepatocellular carcinoma: a systematic review of randomized trials. Eur J Surg Oncol, 2012, 38(4): 286-295.
- 77. Wei W, Jian PE, Li SH, et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety. Cancer Commun (Lond), 2018, 38(1): 61.
- 78. 程樹群, 吳孟超, 陳漢, 等. 肝癌患者術后肝動脈化療栓塞聯合胸腺肽治療預防復發的隨機對照研究. 中華腫瘤雜志, 2004, 26(5): 305-307.
- 79. Gish RG, Gordon SC, Nelson D, et al. A randomized controlled trial of thymalfasin plus transarterial chemoembolization for unresectable hepatocellular carcinoma. Hepatol Int, 2009, 3(3): 480-489.
- 80. He C, Peng W, Li C, et al. Thymalfasin, a promising adjuvant therapy in small hepatocellular carcinoma after liver resection. Medicine (Baltimore), 2017, 96(16): e6606.
- 81. Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet, 2000, 356(9232): 802-807.
- 82. Ikeda K, Arase Y, Saitoh S, et al. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor—A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology, 2000, 32(2): 228-232.
- 83. Sun HC, Tang ZY, Wang L, et al. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol, 2006, 132(7): 458-465.
- 84. Lo CM, Liu CL, Chan SC, et al. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg, 2007, 245(6): 831-842.
- 85. Breitenstein S, Dimitroulis D, Petrowsky H, et al. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg, 2009, 96(9): 975-981.
- 86. Wang SN, Chuang SC, Lee KT. Efficacy of sorafenib as adjuvant therapy to prevent early recurrence of hepatocellular carcinoma after curative surgery: a pilot study. Hepatol Res, 2014, 44(5): 523-531.
- 87. Zhang W, Zhao G, Wei K, et al. Adjuvant sorafenib reduced mortality and prolonged overall survival and post-recurrence survival in hepatocellular carcinoma patients after curative resection: a single-center experience. Biosci Trends, 2014, 8(6): 333-338.
- 88. Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol, 2015, 16(13): 1344-1354.
- 89. Kudo M, Finn RS, Qin SK, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet, 2018, 391(10126): 1163-1173.
- 90. Hiraoka A, Kumada T, Kariyama K, et al. Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: Multicenter analysis. Cancer Med, 2019, 8(1): 137-146.
- 91. Mizuta T, Ozaki I, Eguchi Y, et al. The Effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: a pilot study. Cancer, 2006, 106(4): 867-872.
- 92. Kakizaki S, Sohara N, Sato K, et al. Preventive effects of vitamin K on recurrent disease in patients with hepatocellular carcinoma arising from hepatitis C viral infection. J Gastroenterol Hepatol, 2007, 22(4): 518-522.
- 93. Yoshida H, Shiratori Y, Kudo M, et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology, 2011, 54(2): 532-540.
- 94. Okita K, Izumi N, Matsui O, et al. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: a randomized double-blind placebo-controlled study. J Gastroenterol, 2015, 50(2): 191-202.
- 95. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol, 2013, 31(28): 3501-3508.
- 96. Xia Y, Qiu Y, Li J, et al. Adjuvant therapy with capecitabine postpones recurrence of hepatocellular carcinoma after curative resection: a randomized controlled trial. Ann Surg Oncol, 2010, 17(12): 3137-3144.
- 97. Sun HC, Zhang W, Qin LX, et al. Positive serum hepatitis B E antigen is associated with higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B-related hepatocellular carcinoma. J Hepatol, 2007, 47(5): 684-690.
- 98. Huang G, Lau WY, Wang ZG, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg, 2015, 261(1): 56-66.
- 99. Wong JS, Wong GL, Tsoi KK, et al. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther, 2011, 33(10): 1104-1112.
- 100. Zhou XD, Tang ZY, Ma ZC, et al. Twenty-year survivors after resection for hepatocellular carcinoma-analysis of 53 cases. J Cancer Res Clin Oncol, 2009, 135(8): 1067-1072.
- 101. Si MS, Amersi F, Golish SR, et al. Prevalence of metastases in hepatocellular carcinoma: risk factors and impact on survival. Am Surg, 2003, 69(10): 879-885.
- 102. Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol, 2005, 20(11): 1781-1787.
- 103. Yang Y, Nagano H, Ota H, et al. Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery, 2007, 141(2): 196-202.
- 104. Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer, 2000, 89(3): 500-507.
- 105. Lee KF, Chong CCN, Fong AKW, et al. Pattern of disease recurrence and its implications for postoperative surveillance after curative hepatectomy for hepatocellular carcinoma: experience from a single center. Hepatobiliary Surg Nutr, 2018, 7(5): 320-330.
- 106. Sakamoto M, Hirohashi S, Tsuda H, et al. Multicentric independent development of hepatocellular carcinoma revealed by analysis of hepatitis B virus integration pattern. Am J Surg Pathol, 1989, 13(12): 1064-1067.
- 107. Liver Cancer Study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer. Jpn J Surg, 1989, 19(1): 98-129.
- 108. Takenaka K, Adachi E, Nishizaki T, et al. Possible multicentric occurrence of hepatocellular carcinoma: a clinicopathological study. Hepatology, 1994, 19(4): 889-894.
- 109. Ng IO, Guan XY, Poon RT, et al. Determination of the molecular relationship between multiple tumour nodules in hepatocellular carcinoma differentiates multicentric origin from intrahepatic metastasis. J Pathol, 2003, 199(3): 345-353.
- 110. Chen PJ, Chen DS, Lai MY, et al. Clonal origin of recurrent hepatocellular carcinomas. Gastroenterology, 1989, 96(2 Pt 1): 527-529.
- 111. Hodges KB, Cummings OW, Saxena R, et al. Clonal origin of multifocal hepatocellular carcinoma. Cancer, 2010, 116(17): 4078-4085.
- 112. Wang B, Xia CY, Lau WY, et al. Determination of clonal origin of recurrent hepatocellular carcinoma for personalized therapy and outcomes evaluation: a new strategy for hepatic surgery. J Am Coll Surg, 2013, 217(6): 1054-1062.
- 113. Morimoto O, Nagano H, Sakon M, et al. Diagnosis of intrahepatic metastasis and multicentric carcinogenesis by microsatellite loss of heterozygosity in patients with multiple and recurrent hepatocellular carcinomas. J Hepatol, 2003, 39(2): 215-221.
- 114. Esumi M, Aritaka T, Arii M, et al. Clonal origin of human hepatoma determined by integration of hepatitis B virus DNA. Cancer Res, 1986, 46(11): 5767-5771.
- 115. Iizuka N, Oka M, Yamada-Okabe H, et al. Oligonucleotide microarray for prediction of early intrahepatic recurrence of hepatocellular carcinoma after curative resection. Lancet, 2003, 361(9361): 923-929.
- 116. Cheung ST, Chen X, Guan XY, et al. Identify metastasis-associated genes in hepatocellular carcinoma through clonality delineation for multinodular tumor. Cancer Res, 2002, 62(16): 4711-4721.
- 117. Barry CT, D'Souza M, McCall M, et al. Micro RNA expression profiles as adjunctive data to assess the risk of hepatocellular carcinoma recurrence after liver transplantation. Am J Transplant, 2012, 12(2): 428-437.
- 118. Wilkens L, Bredt M, Flemming P, et al. Differentiation of multicentric origin from intra-organ metastatic spread of hepatocellular carcinomas by comparative genomic hybridization. J Pathol, 2000, 192(1): 43-51.
- 119. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med, 2012, 366(10): 883-892.
- 120. Miao R, Luo H, Zhou H, et al. Identification of prognostic biomarkers in hepatitis B virus-related hepatocellular carcinoma and stratification by integrative multi-omics analysis. J Hepatol, 2014, 61(4): 840-849.
- 121. Xu XF, Xing H, Han J, et al. Risk Factors, Patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg, 2019, 154(3): 209-217.
- 122. Xing H, Zhang WG, Cescon M, et al. Defining and predicting early recurrence after liver resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford), 2020, 22(5): 677-689.
- 123. Erridge S, Pucher PH, Markar SR, et al. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg, 2017, 104(11): 1433-1442.
- 124. Meniconi RL, Komatsu S, Perdigao F, et al. Recurrent hepatocellular carcinoma: a western strategy that emphasizes the impact of pathologic profile of the first resection. Surgery, 2015, 157(3): 454-462.
- 125. Zhang X, Li C, Wen T, et al. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol, 2015, 27(8): 933-940.
- 126. Wang DY, Liu L, Qi XS, et al. Hepatic re-resection versus transarterial chemoembolization for the treatment of recurrent hepatocellular carcinoma after initial resection: a systematic review and meta-analysis. Asian Pac J Cancer Prev, 2015, 16(13): 5573-5578.
- 127. Mise Y, Hasegawa K, Shindoh J, et al. The feasibility of third or more repeat hepatectomy for recurrent hepatocellular carcinoma. Ann Surg, 2015, 262(2): 347-357.
- 128. Yamashita Y, Shirabe K, Tsuijita E, et al. Third or more repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery, 2013, 154(5): 1038-1045.
- 129. Wu CC, Cheng SB, Yeh DC, et al. Second and third hepatectomies for recurrent hepatocellular carcinoma are justified. Br J Surg, 2009, 96(9): 1049-1057.
- 130. Poon RT, Fan ST, O'Suilleabhain CB, et al. Aggressive management of patients with extrahepatic and intrahepatic recurrences of hepatocellular carcinoma by combined resection and locoregional therapy. J Am Coll Surg, 2002, 195(3): 311-318.
- 131. Minagawa M, Makuuchi M, Takayama T, et al. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg, 2003, 238(5): 703-710.
- 132. Zou Q, Li J, Wu D, et al. Nomograms for pre-operative and post-operative prediction of long-term survival of patients who underwent repeat hepatectomy for recurrent hepatocellular carcinoma. Ann Surg Oncol, 2016, 23(8): 2618-2626.
- 133. Yamashita S, Aoki T, Inoue Y, et al. Outcome of salvage hepatic resection for recurrent hepatocellular carcinoma after radiofrequency ablation therapy. Surgery, 2015, 157(3): 463-472.
- 134. Nakajima Y, Ko S, Kanamura T, et al. Repeat liver resection for hepatocellular carcinoma. J Am Coll Surg, 2001, 192(3): 339-344.
- 135. 董家鴻, 鄭樹森, 陳孝平, 等. 肝切除術前肝臟儲備功能評估的專家共識(2011 版). 中華消化外科雜志, 2011, 10(1): 20-25.
- 136. Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology, 1997, 26(5): 1176-1181.
- 137. Shirabe K, Shimada M, Gion T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg, 1999, 188(3): 304-309.
- 138. Shindoh J, D Tzeng CW, Vauthey JN. Portal vein embolization for hepatocellular carcinoma. Liver Cancer, 2012, 1(3-4): 159-167.
- 139. Siriwardana RC, Lo CM, Chan SC, et al. Role of portal vein embolization in hepatocellular carcinoma management and its effect on recurrence: a case-control study. World J Surg, 2012, 36(7): 1640-1646.
- 140. Tsujita E, Utsunomiya T, Ohta M, et al. Outcome of repeat hepatectomy in patients with hepatocellular carcinoma aged 75 years and older. Surgery, 2010, 147(5): 696-703.
- 141. Liu K, Chen Y, Wu X, et al. Laparoscopic liver re-resection is feasible for patients with posthepatectomy hepatocellular carcinoma recurrence: a propensity score matching study. Surg Endosc, 2017, 31(11): 4790-4798.
- 142. Chan AC, Poon RT, Chok KS, et al. Feasibility of laparoscopic re-resection for patients with recurrent hepatocellular carcinoma. World J Surg, 2014, 38(5): 1141-1146.
- 143. Hu M, Zhao G, Xu D, et al. Laparoscopic repeat resection of recurrent hepatocellular carcinoma. World J Surg, 2011, 35(3): 648-655.
- 144. Zhang J, Zhou ZG, Huang ZX, et al. Prospective, single-center cohort study analyzing the efficacy of complete laparoscopic resection on recurrent hepatocellular carcinoma. Chin J Cancer, 2016, 35: 25.
- 145. Kanazawa A, Tsukamoto T, Shimizu S, et al. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Sci, 2013, 20(5): 512-517.
- 146. Belli G, Cioffi L, Fantini C, et al. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: feasibility, safety, and results. Surg Endosc, 2009, 23(8): 1807-1811.
- 147. Halls MC, Cipriani F, Berardi G, et al. Conversion for unfavorable intraoperative events results in significantly worse outcomes during laparoscopic liver resection: lessons learned from a multicenter review of 2861 cases. Ann Surg, 2018, 268(6): 1051-1057.
- 148. Fan J, Wu ZQ, Zhou J, et al. Hepatocellular carcinoma associated with tumor thrombosis in the portal vein: the effects of different treatments. Hepatobiliary Pancreat Dis Int, 2003, 2(4): 513-519.
- 149. Ye JZ, Wang YY, Bai T, et al. Surgical resection for hepatocellular carcinoma with portal vein tumor thrombus in the asia-pacific region beyond the barcelona clinic liver cancer treatment algorithms: a review and update. Oncotarget, 2017, 8(54): 93258-93278.
- 150. Tantawi B, Cherqui D, Tran van Nhieu J, et al. Surgery for biliary obstruction by tumour thrombus in primary liver cancer. Br J Surg, 1996, 83(11): 1522-1525.
- 151. Qin LX, Ma ZC, Wu ZQ, et al. Diagnosis and surgical treatments of hepatocellular carcinoma with tumor thrombosis in bile duct: experience of 34 patients. World J Gastroenterol, 2004, 10(10): 1397-1401.
- 152. Satoh S, Ikai I, Honda G, et al. Clinicopathologic evaluation of hepatocellular carcinoma with bile duct thrombi. Surgery, 2000, 128(5): 779-783.
- 153. Liu QY, Lai DM, Liu C, et al. A Special recurrent pattern in small hepatocellular carcinoma after treatment: bile duct tumor thrombus formation. World J Gastroenterol, 2011, 17(43): 4817-4824.
- 154. Hanaoka J, Shimada M, Ikegami T, et al. Hepatocellular carcinoma with massive bile duct tumor thrombus: report of a long-term survival. Hepatogastroenterology, 2008, 55(88): 2217-2220.
- 155. Moon DB, Hwang S, Wang HJ, et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: a Korean multicenter study. World J Surg, 2013, 37(2): 443-451.
- 156. Wong TC, Cheung TT, Chok KS, et al. Outcomes of hepatectomy for hepatocellular carcinoma with bile duct tumour thrombus. HPB (Oxford), 2015, 17(5): 401-408.
- 157. Xu LB, Liu C. Hepatocellular carcinoma with bile duct tumor thrombus: extrahepatic bile duct preserving or not? Ann Surg, 2017, 266(6): e62-e63.
- 158. Chen XP, Wu ZD, Huang ZY, et al. Use of hepatectomy and splenectomy to treat hepatocellular carcinoma with cirrhotic hypersplenism. Br J Surg, 2005, 92(3): 334-339.
- 159. Zhang X, Li C, Wen T, et al. Synchronous splenectomy and hepatectomy for patients with small hepatocellular carcinoma and pathological spleen: neutrophil to lymphocyte ratio changes can predict the prognosis. Oncotarget, 2017, 8(28): 46298-46311.
- 160. Zhang XY, Li C, Wen TF, et al. Synchronous splenectomy and hepatectomy for patients with hepatocellular carcinoma and hypersplenism: a case-control study. World J Gastroenterol, 2015, 21(8): 2358-2366.
- 161. Zhang Y, Wen TF, Yan LN, et al. Preoperative predictors of portal vein thrombosis after splenectomy with periesophagogastric devascularization. World J Gastroenterol, 2012, 18(15): 1834-1839.
- 162. 張曉赟, 李川, 彭偉, 等. 血小板/脾臟體積比預測肝硬化門靜脈高壓癥的嚴重程度. 腹部外科, 2016, 29(3): 187-192.
- 163. 張宇, 文天夫, 陳哲宇, 等. 術前門靜脈血流速度在門靜脈高壓癥斷流術后血栓形成中的預測價值. 中華外科雜志, 2009, 47(11): 825-828.
- 164. Lu MD, Yin XY, Xie XY, et al. Percutaneous thermal ablation for recurrent hepatocellular carcinoma after hepatectomy. Br J Surg, 2005, 92(11): 1393-1398.
- 165. Thomasset SC, Dennison AR, Garcea G. Ablation for recurrent hepatocellular carcinoma: a systematic review of clinical efficacy and prognostic factors. World J Surg, 2015, 39(5): 1150-1160.
- 166. Chen HW, Lai EC, Zhen ZJ, et al. Ultrasound-guided percutaneous cryotherapy of hepatocellular carcinoma. Int J Surg, 2011, 9(2): 188-191.
- 167. Chen R, Gan Y, Ge N, et al. Transarterial chemoembolization versus radiofrequency ablation for recurrent hepatocellular carcinoma after resection within barcelona clinic liver cancer stage 0/a: a retrospective comparative study. J Vasc Interv Radiol, 2016, 27(12): 1829-1836.
- 168. Wang K, Liu G, Li J, et al. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol, 2015, 41(2): 236-242.
- 169. 段紀成, 劉凱, 吳孟超, 等. 經皮肝穿刺射頻消融與再次肝切除對復發性小肝癌的隨機對照研究. 肝膽外科雜志, 2015, 23(1): 15-17.
- 170. Chan AC, Poon RT, Cheung TT, et al. Survival analysis of re-resection versus radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. World J Surg, 2012, 36(1): 151-156.
- 171. Ho CM, Lee PH, Shau WY, et al. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery, 2012, 151(5): 700-709.
- 172. Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg, 2006, 243(3): 321-328.
- 173. Huang JW, Yan LN, Cheng ZY, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg, 2010, 252(6): 903-912.
- 174. 中國抗癌協會肝癌專業委員會, 中國抗癌協會臨床腫瘤學協作專業委員會, 中華醫學會肝病學分會肝癌學組. 肝癌局部消融治療規范的專家共識. 實用肝臟病雜志, 2011, 14(4): 243-245.
- 175. Liang HH, Chen MS, Peng ZW, et al. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol, 2008, 15(12): 3484-3493.
- 176. Chen MH, Yang W, Yan K, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients—mathematic model, overlapping mode, and electrode placement process. Radiology, 2004, 232(1): 260-271.
- 177. Zhang L, Wang N, Shen Q, et al. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS One, 2013, 8(10): e76119.
- 178. Abdelaziz A, Elbaz T, Shousha HI, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an egyptian multidisciplinary clinic experience. Surg Endosc, 2014, 28(12): 3429-3434.
- 179. Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology, 2012, 262(1): 43-58.
- 180. Farina L, Weiss N, Nissenbaum Y, et al. Characterisation of tissue shrinkage during microwave thermal ablation. Int J Hyperthermia, 2014, 30(7): 419-428.
- 181. Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia, 2016, 32(3): 339-344.
- 182. Luo W, Zhang Y, He G, et al. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol, 2017, 15(1): 126.
- 183. Ei S, Hibi T, Tanabe M, et al. Cryoablation provides superior local control of primary hepatocellular carcinomas of >2 cm compared with radiofrequency ablation and microwave coagulation therapy: an underestimated tool in the toolbox. Ann Surg Oncol, 2015, 22(4): 1294-1300.
- 184. Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology, 2015, 61(5): 1579-1590.
- 185. Chan AC, Cheung TT, Fan ST, et al. Survival analysis of high-intensity focused ultrasound therapy versus radiofrequency ablation in the treatment of recurrent hepatocellular carcinoma. Ann Surg, 2013, 257(4): 686-692.
- 186. Giorgio A, Di Sarno A, De Stefano G, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Res, 2011, 31(6): 2291-2295.
- 187. Brunello F, Veltri A, Carucci P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand J Gastroenterol, 2008, 43(6): 727-735.
- 188. Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology, 2005, 129(1): 122-130.
- 189. Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology, 2003, 228(1): 235-240.
- 190. Yong CC, Tsai MC, Lin CC, et al. Comparison of salvage living donor liver transplantation and local regional therapy for recurrent hepatocellular carcinoma. World J Surg, 2016, 40(10): 2472-2480.
- 191. Chan AC, Chan SC, Chok KS, et al. Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl, 2013, 19(4): 411-419.
- 192. Yamashita Y, Yoshida Y, Kurihara T, et al. Surgical results for recurrent hepatocellular carcinoma after curative hepatectomy: repeat hepatectomy versus salvage living donor liver transplantation. Liver Transpl, 2015, 21(7): 961-968.
- 193. Del Gaudio M, Ercolani G, Ravaioli M, et al. Liver transplantation for recurrent hepatocellular carcinoma on cirrhosis after liver resection: university of bologna experience. Am J Transplant, 2008, 8(6): 1177-1185.
- 194. Lee HS, Choi GH, Joo DJ, et al. The clinical behavior of transplantable recurrent hepatocellular carcinoma after curative resection: implications for salvage liver transplantation. Ann Surg Oncol, 2014, 21(8): 2717-2724.
- 195. Majno PE, Sarasin FP, Mentha G, et al. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology, 2000, 31(4): 899-906.
- 196. Hu Z, Zhou J, Li Z, et al. Time interval to recurrence as a predictor of overall survival in salvage liver transplantation for patients with hepatocellular carcinoma associated with hepatitis B virus. Surgery, 2015, 157(2): 239-248.
- 197. Guerrini GP, Gerunda GE, Montalti R, et al. Results of salvage liver transplantation. Liver Int, 2014, 34(6): e96-e104.
- 198. Hu RH, Ho MC, Wu YM, et al. Feasibility of salvage liver transplantation for patients with recurrent hepatocellular carcinoma. Clin Transplant, 2005, 19(2): 175-180.
- 199. Kaido T, Mori A, Ogura Y, et al. Living donor liver transplantation for recurrent hepatocellular carcinoma after liver resection. Surgery, 2012, 151(1): 55-60.
- 200. Sala M, Fuster J, Llovet JM, et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl, 2004, 10(10): 1294-1300.
- 201. Ferrer-Fàbrega J, Forner A, Liccioni A, et al. Prospective validation of Ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology, 2016, 63(3): 839-849.
- 202. Yang PC, Ho CM, Hu RH, et al. Prophylactic liver transplantation for high-risk recurrent hepatocellular carcinoma. World J Hepatol, 2016, 8(31): 1309-1317.
- 203. Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology, 2002, 35(5): 1164-1171.
- 204. Llovet JM, Real MI, Monta?a X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet, 2002, 359(9319): 1734-1739.
- 205. Jin YJ, Chung YH, Kim JA, et al. Predisposing factors of hepatocellular carcinoma recurrence following complete remission in response to transarterial chemoembolization. Dig Dis Sci, 2013, 58(6): 1758-1765.
- 206. Wang Q, Xia D, Bai W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: a multicentre observational study. J Hepatol, 2019, 70(5): 893-903.
- 207. Koh PS, Chan AC, Cheung TT, et al. Efficacy of radiofrequency ablation compared with transarterial chemoembolization for the treatment of recurrent hepatocellular carcinoma: a comparative survival analysis. HPB (Oxford), 2016, 18(1): 72-78.
- 208. Cheng YC, Chen TW, Fan HL, et al. Transarterial chemo-embolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant, 2014, 19: 309-316.
- 209. Jin YJ, Lee JW, Lee OH, et al. Transarterial chemoembolization versus surgery/radiofrequency ablation for recurrent hepatocellular carcinoma with or without microvascular invasion. J Gastroenterol Hepatol, 2014, 29(5): 1056-1064.
- 210. Yang W, Chen MH, Wang MQ, et al. Combination therapy of radiofrequency ablation and transarterial chemoembolization in recurrent hepatocellular carcinoma after hepatectomy compared with single treatment. Hepatol Res, 2009, 39(3): 231-240.
- 211. Dai WC, Cheung TT. Strategic overview on the best treatment option for intrahepaitc hepatocellular carcinoma recurrence. Expert Rev Anticancer Ther, 2016, 16(10): 1063-1072.
- 212. 曾昭沖. 消融治療后復發肝癌及肝外轉移的放射治療. 中華醫學雜志, 2015, 95(27): 2164-2166.
- 213. Bae SH, Park HC, Lim DH, et al. Salvage treatment with hypofractionated radiotherapy in patients with recurrent small hepatocellular carcinoma. Int J Radiat Oncol Biol Phys, 2012, 82(4): e603-607.
- 214. Su TS, Liang P, Lu HZ, et al. Stereotactic body radiation therapy for small primary or recurrent hepatocellular carcinoma in 132 Chinese patients. J Surg Oncol, 2016, 113(2): 181-187.
- 215. Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol, 2014, 53(3): 399-404.
- 216. Seo YS, Kim MS, Yoo HJ, et al. Radiofrequency ablation versus stereotactic body radiotherapy for small hepatocellular carcinoma: a markov model-based analysis. Cancer Med, 2016, 5(11): 3094-3101.
- 217. Nakazawa T, Adachi S, Kitano M, et al. Potential prognostic benefits of radiotherapy as an initial treatment for patients with unresectable advanced hepatocellular carcinoma with invasion to intrahepatic large vessels. Oncology, 2007, 73(1-2): 90-97.
- 218. Otsuka M, Ohara K, Takada Y, et al. Radiation therapy for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. Int J Clin Oncol, 2003, 8(3): 151-155.
- 219. Huang WY, Jen YM, Lee MS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys, 2012, 84(2): 355-361.
- 220. He J, Zeng ZC, Tang ZY, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer, 2009, 115(12): 2710-2720.
- 221. Chang UK, Kim MS, Han CJ, et al. Clinical result of stereotactic radiosurgery for spinal metastasis from hepatocellular carcinoma: comparison with conventional radiation therapy. J Neurooncol, 2014, 119(1): 141-148.
- 222. Byun HK, Kim HJ, Im YR, et al. Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma. Radiother Oncol, 2019, 133: 1-8.
- 223. 中華醫學會放射腫瘤學分會, 中國生物醫學工程學會精確放療分會, 肝癌學組與消化系統腫瘤專家委員會, 等. 2016 年原發性肝癌放療共識. 中華放射腫瘤學雜志, 2016, 25(11): 1141-1150.
- 224. Rivera L, Giap H, Miller W, et al. Hepatic intra-arterial infusion of yttrium-90 microspheres in the treatment of recurrent hepatocellular carcinoma after liver transplantation: a case report. World J Gastroenterol, 2006, 12(35): 5729-5732.
- 225. 中華醫學會核醫學分會轉移性骨腫瘤治療工作委員會. 氯化鍶[89Sr]治療轉移性骨腫瘤專家共識(2017 年版). 中華核醫學與分子影像雜志, 2018, 38(6): 412-415.
- 226. Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology, 2016, 151(6): 1155-1163.
- 227. Peng ZW, Zhang YJ, Liang HH, et al. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and rf ablation versus rf ablation alone: a prospective randomized trial. Radiology, 2012, 262(2): 689-700.
- 228. Khan MA, Combs CS, Brunt EM, et al. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol, 2000, 32(5): 792-797.
- 229. Ali SA, Amin DH, Abdelkhalek YI. Efficiency of whole-body 18F-FDG PET CT in detecting the cause of rising serum AFP level in post-therapeutic follow-up for HCC patients. Jpn J Radiol, 2020, 38(5): 472-479.
- 230. Nakano M, Tanaka M, Kuromatsu R, et al. Sorafenib for the treatment of advanced hepatocellular carcinoma with extrahepatic metastasis: a prospective multicenter cohort study. Cancer Med, 2015, 4(12): 1836-1843.
- 231. Sohn W, Paik YH, Cho JY, et al. Sorafenib therapy for hepatocellular carcinoma with extrahepatic spread: treatment outcome and prognostic factors. J Hepatol, 2015, 62(5): 1112-1121.
- 232. Zhang SM, Zeng ZC, Tang ZY, et al. Prognostic analysis of pulmonary metastases from hepatocellular carcinoma. Hepatol Int, 2008, 2(2): 237-243.
- 233. Yoon YS, Kim HK, Kim J, et al. Long-term survival and prognostic factors after pulmonary metastasectomy in hepatocellular carcinoma. Ann Surg Oncol, 2010, 17(10): 2795-2801.
- 234. Chen F, Sato K, Fujinaga T, et al. Pulmonary resection for metastases from hepatocellular carcinoma. World J Surg, 2008, 32(10): 2213-2217.
- 235. Kobayashi S, Takahashi S, Kato Y, et al. Surgical treatment of lymph node metastases from hepatocellular carcinoma. J Hepatobiliary Pancreat Sci, 2011, 18(4): 559-566.
- 236. Ha TY, Hwang S, Ahn CS, et al. Resection of metachronous adrenal metastasis after liver resection and transplantation for hepatocellular carcinoma. Dig Surg, 2014, 31(6): 428-435.
- 237. Kim SU, Kim DY, Park JY, et al. Hepatocellular carcinoma presenting with bone metastasis: clinical characteristics and prognostic factors. J Cancer Res Clin Oncol, 2008, 134(12): 1377-1384.
- 238. Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver Int, 2005, 25(2): 261-265.
- 239. Xu L, Wang J, Kim Y, et al. A randomized controlled trial on patients with or without adjuvant autologous cytokine-induced killer cells after curative resection for hepatocellular carcinoma. Oncoimmunology, 2015, 5(3): e1083671.
- 240. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase Ⅲ trial. J Clin Oncol, 2020, 38(3): 193-202.
- 241. Sangro B, Park JW, Cruz D, et al. A randomized, multicenter, phase 3 study of nivolumab vs sorafenib as first-line treatment in patients (pts) with advanced hepatocellular carcinoma (HCC): CheckMate-459. J Clin Oncol, 2016, 34(15 Suppl): TPS4147.
- 242. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med, 2020, 382(20): 1894-1905.
- 243. Qin SK, Ren ZG, Meng ZQ, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol, 2020, 21(4): 571-580.
- 244. Chen Q, Shu C, Laurence AD, et al. Effect of huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut, 2018, 67(11): 2006-2016.