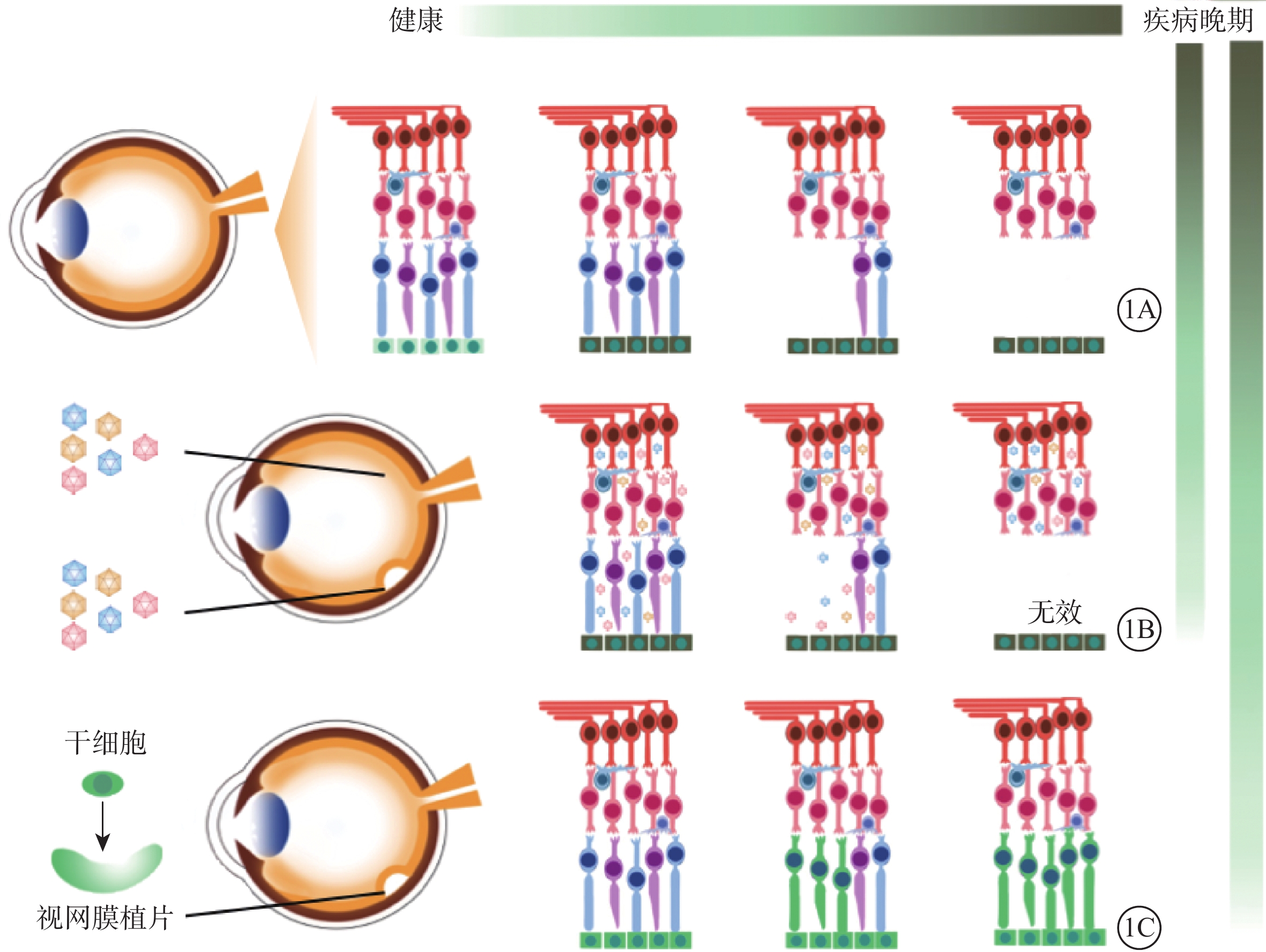
遺傳性眼底病是造成患者視力不可逆損害的一重要原因,因其預后差、臨床缺乏有效干預手段而備受關注。隨著大量遺傳性眼底病致病基因的發現以及基因編輯技術與干細胞技術的發展,基因與干細胞治療應運而生,成為治愈這類疾病的新希望。基因治療更多針對早期遺傳性眼底病,以導入野生型基因片段替代突變基因來維持現有的視網膜細胞活力;干細胞治療則更多地針對晚期遺傳性眼底病,以健康的干細胞來置換和填充失去功能的視網膜細胞。雖然基因與干細胞治療仍面臨基因脫靶、分化效率、細胞遷移、長期療效等諸多問題,但其在臨床前期及臨床試驗中取得的成果不容小覷。隨著各種新技術和新材料的出現,勢必進一步輔助基因與干細胞治療策略,為遺傳性眼底病的臨床治愈帶來無限機遇和無限可能。
引用本文: 韓如意, 金子兵. 遺傳性眼底病基因和干細胞治療趨勢與面臨的挑戰和機遇. 中華眼底病雜志, 2018, 34(6): 519-525. doi: 10.3760/cma.j.issn.1005-1015.2018.06.001 復制
視網膜色素變性(RP)、先天性黑矇(LCA)、先天性靜止性夜盲、結晶樣視網膜變性、Stargardt病等遺傳性眼底病是中青年致盲的主要病因[1]。因其預后差、臨床上缺乏有效干預手段而備受關注。隨著研究技術的進步以及學者們的不懈努力,目前已發現超過250個遺傳性眼底病的致病基因[2]。其中單就RP而言,就有超過近5000個突變位點及相關的80余個基因被報道[3]。眼球獨特的解剖結構和相對免疫豁免特點以及眼結構、功能檢測手段的發展為基因和干細胞治療奠定了基礎[4-5]。基因治療和干細胞治療策略應運而生,成為了治愈這類疾病的新希望。部分RP常以視網膜色素上皮(RPE)功能失代償為原發起始,隨后逐漸出現光感受器細胞凋亡(圖1A)。因此,隨疾病發展,理想的治療策略也相應改變。基因治療更多針對早期遺傳性眼底病,以導入野生型基因片段替代突變基因來維持現有的視網膜細胞活力(圖1B);干細胞治療則更多地針對晚期遺傳性眼底病,以健康的干細胞來置換和填充失去功能的視網膜細胞[6-7](圖1C)。
1 基因治療走進臨床
在遺傳性眼底病早期,視網膜細胞凋亡數目較少,現存的細胞仍可維持患者部分視功能。若對現存細胞進行基因編輯,可以很大程度地提高細胞活力,改善視功能。目前的基因治療策略主要為基因增補或合并基因抑制策略。對于隱性突變而言,導入野生型基因片段替代突變基因片段表達蛋白便可改善表型。因此,在RPE65基因突變的LCA、常染色體隱性RP(arRP)及X連鎖RP(XLRP)等疾病中,此策略都取得了極大成功。例如,RPE65基因突變的犬和小鼠接受腺相關病毒(AAV)介導導入的野生型RPE65基因片段后,其短期及長期(6個月~3年)監測結果均顯示視網膜電圖(ERG)及視銳度有明顯改善,且無明顯的毒性反應[8-17]。以動物實驗為基礎,2008年先后有多個團隊報道了RPE65基因突變的成年LCA患者接受基因治療后短期及長期觀測中,患者均未發生嚴重免疫反應或其他不良事件,且其視覺敏感度均有不同程度提高[18-21]。2016年,11例兒童及成年LCA患者在一側眼已經接受過基因治療的情況下,再次接受對側眼RPE65基因治療,其結果證明了此基因療法的安全性[22]。2017年,Russell等[23]首次對31例患者進行了隨機雙盲對照3期臨床試驗,數據顯示RPE65基因治療可顯著提高患者的多項視功能。2017年10月,首項治療RPE65基因缺陷的藥物Luxturna獲得美國食品與藥品管理局批準,成功進入市場。另外,由CNGB1或MERTK基因突變造成的arRP小鼠、大鼠及犬模型,經視網膜下腔注射AAV介導的野生型基因片段后,其視桿細胞或RPE細胞中相應野生型基因表達量上調,光感受器細胞凋亡速度減慢;并且,在18個月的長期觀察中,其提高的ERG振幅仍得以維持[24-28]。同樣的,在RPGR基因突變引起的XLRP小鼠及犬突變模型中導入野生型人RPGR基因片段以及人IRBP或GRK1啟動子后,短期(2個月)及長期(>2年)監測結果同樣顯示光感受器細胞結構和功能得以改善和保存[29-32]。
對于顯性突變而言,突變基因編碼的蛋白為功能獲得性(gain-of-function)有害蛋白。單純導入野生型基因片段只能部分稀釋而不能從根本上消除這樣的有害效應[33]。于是學者們提出合并基因抑制的策略,主要為DNA水平上直接敲除突變基因、RNA水平上干擾突變基因轉錄或翻譯等。而這樣的策略也在多種動物模型中得以驗證。以CRISPR/Cas9基因編輯技術和siRNA為例,利用電轉技術直接將guide RNA與Cas9的質粒或蛋白(或合并供體cDNA片段)導入視網膜細胞,對突變基因進行特異性DNA雙鏈剪切,實現基因敲除(或)敲入。利用此技術對RHO基因突變大鼠進行基因敲除,成功減慢視網膜凋亡速度,提高視功能[34-36]。利用AAV載體介導的siRNA可使RHO+/?雜合小鼠及犬體內突變基因的mRNA表達量明顯下調(>98.5%)[37-38]。合并導入有抵抗干擾能力的野生型RHO基因片段可明顯改善表型,包括光感受器細胞超微結構恢復、外核層厚度明顯增加、對應的ERG振幅提高等[2, 4, 39-41]。而在遺傳性視神經病變、視錐細胞營養不良、無脈絡膜癥、青年性視網膜劈裂癥等其他遺傳性眼底病中,基因增補策略或是合并基因抑制策略也都在相應動物模型和臨床試驗中取得佳績[42-45]。
同時,介導基因治療的載體及注射方法也得到了諸多關注和研究。載體中AAV載體因非致病原性、低免疫原性、穩定性以及能感染多種類型視網膜細胞等特點尤為受到偏愛[42, 46];但其局限性在于基因負載能力小(AAV<5.2 kb of genetic cargo)、安全性問題等[47-48]。因此,新型非病毒載體也在不斷挖掘中,目前已被報道的非病毒載體有脂質體、多聚納米材料等[49-54]。對于注射方法而言,早期注射方式主要為視網膜下腔注射。通過眼內的玻璃體切除以及視網膜造孔術將光感受器細胞層與RPE層分離開,再將混合液注入間隙中[55]。該方式的優勢為轉導效率高,能夠有效靶向外層視網膜;但其在臨床運用上有明顯的缺陷,如作用范圍受限于注射位點周邊、有創注射易形成醫源性注射位點的局部視網膜脫離等[56-60]。為了探尋其他損傷較小的方式,學者們嘗試進行玻璃體腔注射。但由于視網膜內界膜的存在,經玻璃體腔注射后,很多AAV亞型表現為低轉導效率,包括AAV1、AAV4、AAV5[61]。唯有AAV2在玻璃體腔注射后能表現出對視網膜神經節細胞(RGC)、光感受器細胞的較高轉導效率[62-64]。對此,主要有如下幾種方案來解決玻璃體腔注射帶來的低效率問題:(1)探索AAV新亞型。Lebherz等[61]通過在小鼠眼內的多次對比試驗,發現AAV7經玻璃體腔注射后能表現出較高的轉導效率。(2)改造原有載體。Martin等[63]對AAV載體進行改造,在其中加入雞β-肌動蛋白啟動子和土撥鼠肝炎轉錄后調控元件,經單次玻璃體腔注射2周后大鼠RGC被轉導的效率高達85%。(3)利用蛋白酶消化內界膜[55]。
基因治療在視網膜疾病上首次取得成功,將逐步應用于臨床。然而由于基因大小、顯性基因等問題,并非所有遺傳性眼底病都可以通過基因治療實現疾病逆轉,這仍需要大量的研究努力。
2 干細胞治療風起云涌
對于晚期遺傳性眼底病,當其光感受器細胞出現廣泛凋亡時,單純的基因治療沒有可作用的“靶細胞”,不能延緩其疾病發展(圖1B)。此時,干細胞治療策略成為首選。通過移植健康的干細胞填充和置換無功能的視網膜細胞以達到治療效果[7],其最大優勢在于干細胞有分化成任何類型細胞的潛能(圖1C)。干細胞包括胚胎干細胞(ESC)、誘導多能干細胞(iPSC)、間充質干細胞(MSC)、臍帶血干細胞和羊水干細胞等[65]。其中以ESCs、iPSCs、MSCs為主要移植材料[66-67]。
自1998年Thomson等[68]首次在人類囊胚中獲得ESC后,其巨大的分化潛能吸引了學者對其進行研究。然而ESC向光感受器細胞分化并不容易。2004年Meyer等[69]將表達增強型綠色熒光蛋白的B5小鼠ESC通過玻璃體腔注射的方式植入視網膜變性小鼠眼內,6周后觀察到移植的ESC能部分整合進受體視網膜內,經視黃酸誘導后ESC可表現出視網膜神經樣細胞的特點,如出現大量膨體、表達神經類細胞和突觸的特異性因子等。2006年同一團隊嘗試先將ESC在體外分化為神經干細胞,再進行體內移植;16周后觀察到神經干細胞成功整合進受體視網膜,無明顯免疫反應或細胞異常增生,但也并未向光感受器細胞分化[70]。同年,Banin等[71]提出可能是微環境影響了ESC的定向分化,并證實視網膜下腔的環境可促使ESC向光感受器細胞分化,表達視蛋白等特異性因子。而將人ESC(hESC)與視網膜變性小鼠視網膜共同培養時,確實可觀察到其向光感受器細胞分化,表達Nrl、Crx等特異性轉錄因子[72]。2008年Osakada等[73]證明,在無視網膜組織存在的情況下添加Notch信號通路抑制劑及視黃酸和牛磺酸也可誘導光感受器細胞形成。隨后ESC起源的視網膜組織也被證實可在rd小鼠視網膜內形成外核層,與受體視網膜形成突觸,并提高視功能[74-75]。
與此相比,ESC向RPE分化顯得較為輕松。在分化培養基中加入煙酰堿等特定因子,hESC可向RPE定向分化[76]。將hESC誘導生成的RPE植入NIHⅢ免疫缺陷小鼠視網膜下腔中,沒有發生細胞異常增生[77]。將其植入視網膜變性或Stargardt病動物模型中,與對側眼相比,實驗眼視網膜結構和功能得以改善或維持[77-79]。以此為基礎,2011年首項干細胞治療臨床試驗得以開展。hESC誘導的RPE植片經視網膜下腔移植入9例Stargardt病患者及9例老年性黃斑變性(AMD)患者眼內,其植片呈典型RPE表型并成功整合入患者RPE層[80]。在短期(4個月)及長期(22個月、4年)觀察中,均未發生異常增生或免疫排斥等不良事件。并且,在短期觀察中患者最佳矯正視力(BCVA)從手動提高至20/800;長期觀察中BCVA較前提高10只眼,維持7只眼,下降1只眼。而對側未治療眼無類似視力提高表現[80-82]。2015年,Song等[83]將hESC-RPE植片應用于2例Stargardt病患者及2例AMD患者,結果同樣證明了ESC治療策略的安全性。
與ESC移植相比,自體iPSC更好地避開了倫理問題及免疫排斥問題。2006年,通過4個轉錄因子(Oct3/4、Sox2、Klf4、c-Myc)的表達,小鼠表皮細胞首次被誘導生成多能干細胞[84]。隨后人iPSC也得以建立,且其在形態、增生、表面抗原、基因表達及端粒酶活性等方面與hESC類似[85-86]。然而,從體細胞誘導成為iPSC過程中低效率、依賴于病毒介導的基因整合等一定程度上限制了iPSC的應用。對此,學者們提出了利用小分子化合物(如丙戊酸、鋰)、非整合型附加型載體、piggyBac轉座子、重組蛋白等多種方案[87-91]。與ESC類似,iPSC的分化方法也多種多樣,如基質細胞共培養、自發分化、重組蛋白與化合物誘導[7, 92]。至此,已有多項患者iPSC得以建立并成功分化為RPE細胞、光感受器細胞等,且被證實其在形態及電生理等功能上與同類細胞相似,并被應用于研究疾病機理及藥物篩選[93-97]。而臨床前試驗及臨床試驗的開展更是為干細胞臨床治療奠定了基礎。2012年,Li等[94]將iPSC誘導生成的RPE細胞注入出生2 d后的視網膜變性小鼠視網膜下腔內,在小鼠的整個生命周期中無腫瘤發生,且在長期觀察中ERG振幅有所提高。與之類似,iPSC誘導的光感受器細胞移植入視網膜變性早期及晚期小鼠及豬的視網膜下腔內,可觀察到移植的細胞整合進視網膜外核層,并生成似外節樣的投射樣形態。小鼠模型中b波振幅提高,光誘導的穿梭回避反應改善,且通過膜電極及多焦ERG分析證實RGC的電生理反應部分來自于植片[98-100]。以動物實驗為基礎,2017年AMD患者的iPSC建立,其誘導的RPE植片通過手術方式植入患者眼內后1年,雖無腫瘤生成,但患者BCVA也無明顯提高[101]。來源于患者的iPSC潛在問題值得重視,未經基因修正的iPSC,其分化出來的視網膜將出現同樣的疾病表型。干細胞移植前的基因修正必不可少。而CRISPR/Cas9技術的出現使基因修正更加簡便。Burnight等[102]利用此技術在iPSC上分別進行了對外顯子突變、剪接位點突變、顯性突變的基因修復,證明未出現編碼區域的基因脫靶。2018年Deng等[103]對RPGR基因突變的RP患者iPSC進行3D分化,觀察到纖毛縮短、異常的光感受器細胞形態、定位、轉錄譜及電生理活動,而經CRISPR/Cas9技術糾正基因突變后,其纖毛形態及基因表達再次得以恢復。這不僅模擬了RPGR基因突變引起的病理生理變化,也證明了CRISPR/Cas9技術在干細胞移植的應用前景。基于iPSC的干細胞治療勢不可擋,同時MSC的臨床試驗也在有序進行。前后有22例RP患者接受玻璃體腔MSC移植后無腫瘤發生;短期(3個月)視力相關的生活質量評估也顯示其生活質量得以提高[104-105]。
干細胞使21世紀再生醫學發生了翻天覆地的變化。與基因治療一樣,干細胞治療也在眼底病上實現了開創性勝利。然而,如何高效分化神經視網膜細胞?尤其是光感受器細胞,如何使移植神經元生存并發揮功能?還需要進一步研究。
3 展望
基因與干細胞治療運用為臨床治療遺傳性眼底病開拓了新道路。雖然面臨基因脫靶、分化效率、細胞遷移、長期療效問題等諸多問題[106-109],但其在臨床前期及臨床試驗中取得的成果不容小覷。而CRISPR/Cas9技術、納米材料等新技術和新材料的出現[110],勢必將進一步輔助基因與干細胞治療策略,為遺傳性眼底病的臨床治愈帶來無限機遇和無限可能。
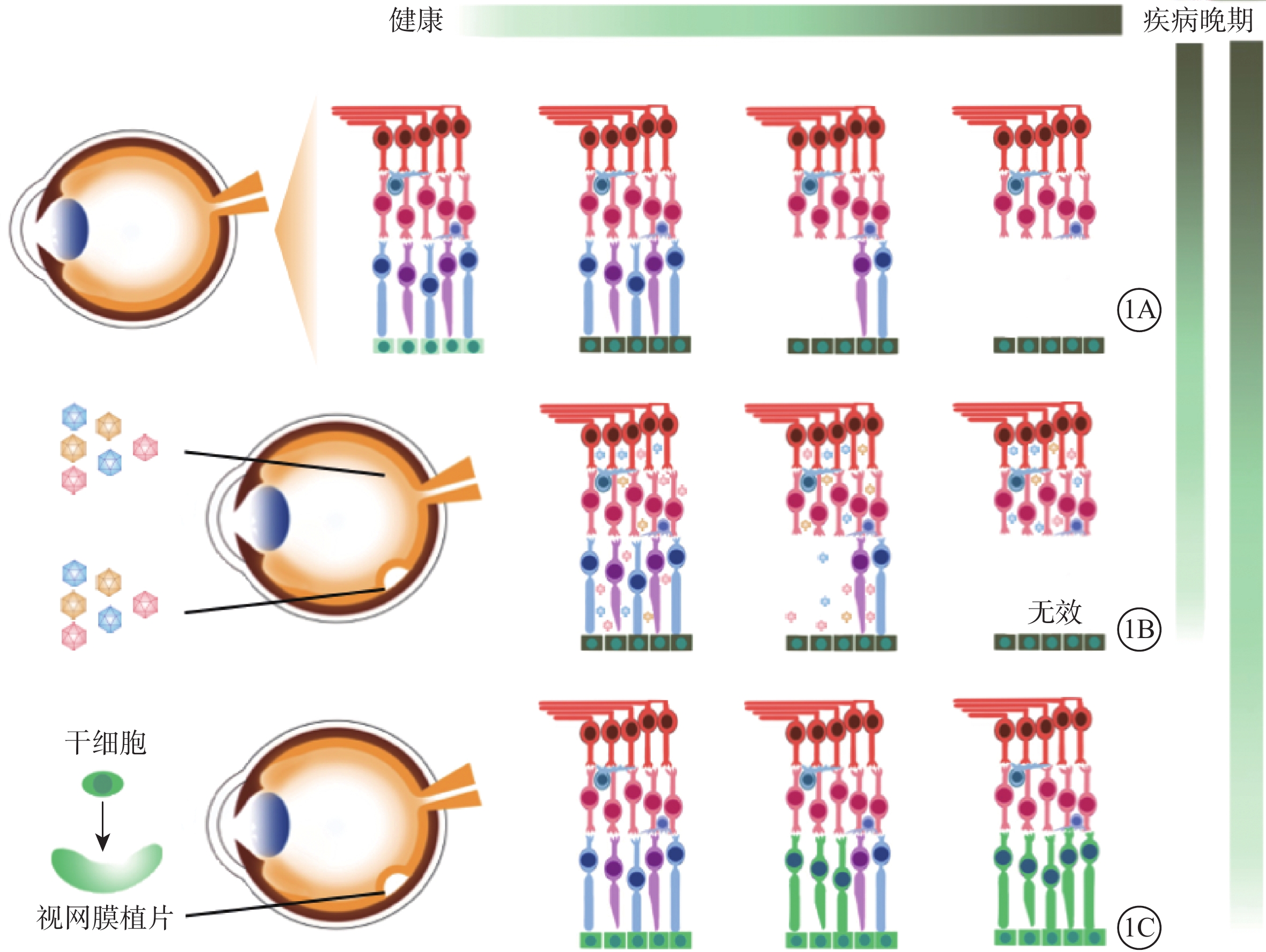
視網膜色素變性(RP)、先天性黑矇(LCA)、先天性靜止性夜盲、結晶樣視網膜變性、Stargardt病等遺傳性眼底病是中青年致盲的主要病因[1]。因其預后差、臨床上缺乏有效干預手段而備受關注。隨著研究技術的進步以及學者們的不懈努力,目前已發現超過250個遺傳性眼底病的致病基因[2]。其中單就RP而言,就有超過近5000個突變位點及相關的80余個基因被報道[3]。眼球獨特的解剖結構和相對免疫豁免特點以及眼結構、功能檢測手段的發展為基因和干細胞治療奠定了基礎[4-5]。基因治療和干細胞治療策略應運而生,成為了治愈這類疾病的新希望。部分RP常以視網膜色素上皮(RPE)功能失代償為原發起始,隨后逐漸出現光感受器細胞凋亡(圖1A)。因此,隨疾病發展,理想的治療策略也相應改變。基因治療更多針對早期遺傳性眼底病,以導入野生型基因片段替代突變基因來維持現有的視網膜細胞活力(圖1B);干細胞治療則更多地針對晚期遺傳性眼底病,以健康的干細胞來置換和填充失去功能的視網膜細胞[6-7](圖1C)。
1 基因治療走進臨床
在遺傳性眼底病早期,視網膜細胞凋亡數目較少,現存的細胞仍可維持患者部分視功能。若對現存細胞進行基因編輯,可以很大程度地提高細胞活力,改善視功能。目前的基因治療策略主要為基因增補或合并基因抑制策略。對于隱性突變而言,導入野生型基因片段替代突變基因片段表達蛋白便可改善表型。因此,在RPE65基因突變的LCA、常染色體隱性RP(arRP)及X連鎖RP(XLRP)等疾病中,此策略都取得了極大成功。例如,RPE65基因突變的犬和小鼠接受腺相關病毒(AAV)介導導入的野生型RPE65基因片段后,其短期及長期(6個月~3年)監測結果均顯示視網膜電圖(ERG)及視銳度有明顯改善,且無明顯的毒性反應[8-17]。以動物實驗為基礎,2008年先后有多個團隊報道了RPE65基因突變的成年LCA患者接受基因治療后短期及長期觀測中,患者均未發生嚴重免疫反應或其他不良事件,且其視覺敏感度均有不同程度提高[18-21]。2016年,11例兒童及成年LCA患者在一側眼已經接受過基因治療的情況下,再次接受對側眼RPE65基因治療,其結果證明了此基因療法的安全性[22]。2017年,Russell等[23]首次對31例患者進行了隨機雙盲對照3期臨床試驗,數據顯示RPE65基因治療可顯著提高患者的多項視功能。2017年10月,首項治療RPE65基因缺陷的藥物Luxturna獲得美國食品與藥品管理局批準,成功進入市場。另外,由CNGB1或MERTK基因突變造成的arRP小鼠、大鼠及犬模型,經視網膜下腔注射AAV介導的野生型基因片段后,其視桿細胞或RPE細胞中相應野生型基因表達量上調,光感受器細胞凋亡速度減慢;并且,在18個月的長期觀察中,其提高的ERG振幅仍得以維持[24-28]。同樣的,在RPGR基因突變引起的XLRP小鼠及犬突變模型中導入野生型人RPGR基因片段以及人IRBP或GRK1啟動子后,短期(2個月)及長期(>2年)監測結果同樣顯示光感受器細胞結構和功能得以改善和保存[29-32]。
對于顯性突變而言,突變基因編碼的蛋白為功能獲得性(gain-of-function)有害蛋白。單純導入野生型基因片段只能部分稀釋而不能從根本上消除這樣的有害效應[33]。于是學者們提出合并基因抑制的策略,主要為DNA水平上直接敲除突變基因、RNA水平上干擾突變基因轉錄或翻譯等。而這樣的策略也在多種動物模型中得以驗證。以CRISPR/Cas9基因編輯技術和siRNA為例,利用電轉技術直接將guide RNA與Cas9的質粒或蛋白(或合并供體cDNA片段)導入視網膜細胞,對突變基因進行特異性DNA雙鏈剪切,實現基因敲除(或)敲入。利用此技術對RHO基因突變大鼠進行基因敲除,成功減慢視網膜凋亡速度,提高視功能[34-36]。利用AAV載體介導的siRNA可使RHO+/?雜合小鼠及犬體內突變基因的mRNA表達量明顯下調(>98.5%)[37-38]。合并導入有抵抗干擾能力的野生型RHO基因片段可明顯改善表型,包括光感受器細胞超微結構恢復、外核層厚度明顯增加、對應的ERG振幅提高等[2, 4, 39-41]。而在遺傳性視神經病變、視錐細胞營養不良、無脈絡膜癥、青年性視網膜劈裂癥等其他遺傳性眼底病中,基因增補策略或是合并基因抑制策略也都在相應動物模型和臨床試驗中取得佳績[42-45]。
同時,介導基因治療的載體及注射方法也得到了諸多關注和研究。載體中AAV載體因非致病原性、低免疫原性、穩定性以及能感染多種類型視網膜細胞等特點尤為受到偏愛[42, 46];但其局限性在于基因負載能力小(AAV<5.2 kb of genetic cargo)、安全性問題等[47-48]。因此,新型非病毒載體也在不斷挖掘中,目前已被報道的非病毒載體有脂質體、多聚納米材料等[49-54]。對于注射方法而言,早期注射方式主要為視網膜下腔注射。通過眼內的玻璃體切除以及視網膜造孔術將光感受器細胞層與RPE層分離開,再將混合液注入間隙中[55]。該方式的優勢為轉導效率高,能夠有效靶向外層視網膜;但其在臨床運用上有明顯的缺陷,如作用范圍受限于注射位點周邊、有創注射易形成醫源性注射位點的局部視網膜脫離等[56-60]。為了探尋其他損傷較小的方式,學者們嘗試進行玻璃體腔注射。但由于視網膜內界膜的存在,經玻璃體腔注射后,很多AAV亞型表現為低轉導效率,包括AAV1、AAV4、AAV5[61]。唯有AAV2在玻璃體腔注射后能表現出對視網膜神經節細胞(RGC)、光感受器細胞的較高轉導效率[62-64]。對此,主要有如下幾種方案來解決玻璃體腔注射帶來的低效率問題:(1)探索AAV新亞型。Lebherz等[61]通過在小鼠眼內的多次對比試驗,發現AAV7經玻璃體腔注射后能表現出較高的轉導效率。(2)改造原有載體。Martin等[63]對AAV載體進行改造,在其中加入雞β-肌動蛋白啟動子和土撥鼠肝炎轉錄后調控元件,經單次玻璃體腔注射2周后大鼠RGC被轉導的效率高達85%。(3)利用蛋白酶消化內界膜[55]。
基因治療在視網膜疾病上首次取得成功,將逐步應用于臨床。然而由于基因大小、顯性基因等問題,并非所有遺傳性眼底病都可以通過基因治療實現疾病逆轉,這仍需要大量的研究努力。
2 干細胞治療風起云涌
對于晚期遺傳性眼底病,當其光感受器細胞出現廣泛凋亡時,單純的基因治療沒有可作用的“靶細胞”,不能延緩其疾病發展(圖1B)。此時,干細胞治療策略成為首選。通過移植健康的干細胞填充和置換無功能的視網膜細胞以達到治療效果[7],其最大優勢在于干細胞有分化成任何類型細胞的潛能(圖1C)。干細胞包括胚胎干細胞(ESC)、誘導多能干細胞(iPSC)、間充質干細胞(MSC)、臍帶血干細胞和羊水干細胞等[65]。其中以ESCs、iPSCs、MSCs為主要移植材料[66-67]。
自1998年Thomson等[68]首次在人類囊胚中獲得ESC后,其巨大的分化潛能吸引了學者對其進行研究。然而ESC向光感受器細胞分化并不容易。2004年Meyer等[69]將表達增強型綠色熒光蛋白的B5小鼠ESC通過玻璃體腔注射的方式植入視網膜變性小鼠眼內,6周后觀察到移植的ESC能部分整合進受體視網膜內,經視黃酸誘導后ESC可表現出視網膜神經樣細胞的特點,如出現大量膨體、表達神經類細胞和突觸的特異性因子等。2006年同一團隊嘗試先將ESC在體外分化為神經干細胞,再進行體內移植;16周后觀察到神經干細胞成功整合進受體視網膜,無明顯免疫反應或細胞異常增生,但也并未向光感受器細胞分化[70]。同年,Banin等[71]提出可能是微環境影響了ESC的定向分化,并證實視網膜下腔的環境可促使ESC向光感受器細胞分化,表達視蛋白等特異性因子。而將人ESC(hESC)與視網膜變性小鼠視網膜共同培養時,確實可觀察到其向光感受器細胞分化,表達Nrl、Crx等特異性轉錄因子[72]。2008年Osakada等[73]證明,在無視網膜組織存在的情況下添加Notch信號通路抑制劑及視黃酸和牛磺酸也可誘導光感受器細胞形成。隨后ESC起源的視網膜組織也被證實可在rd小鼠視網膜內形成外核層,與受體視網膜形成突觸,并提高視功能[74-75]。
與此相比,ESC向RPE分化顯得較為輕松。在分化培養基中加入煙酰堿等特定因子,hESC可向RPE定向分化[76]。將hESC誘導生成的RPE植入NIHⅢ免疫缺陷小鼠視網膜下腔中,沒有發生細胞異常增生[77]。將其植入視網膜變性或Stargardt病動物模型中,與對側眼相比,實驗眼視網膜結構和功能得以改善或維持[77-79]。以此為基礎,2011年首項干細胞治療臨床試驗得以開展。hESC誘導的RPE植片經視網膜下腔移植入9例Stargardt病患者及9例老年性黃斑變性(AMD)患者眼內,其植片呈典型RPE表型并成功整合入患者RPE層[80]。在短期(4個月)及長期(22個月、4年)觀察中,均未發生異常增生或免疫排斥等不良事件。并且,在短期觀察中患者最佳矯正視力(BCVA)從手動提高至20/800;長期觀察中BCVA較前提高10只眼,維持7只眼,下降1只眼。而對側未治療眼無類似視力提高表現[80-82]。2015年,Song等[83]將hESC-RPE植片應用于2例Stargardt病患者及2例AMD患者,結果同樣證明了ESC治療策略的安全性。
與ESC移植相比,自體iPSC更好地避開了倫理問題及免疫排斥問題。2006年,通過4個轉錄因子(Oct3/4、Sox2、Klf4、c-Myc)的表達,小鼠表皮細胞首次被誘導生成多能干細胞[84]。隨后人iPSC也得以建立,且其在形態、增生、表面抗原、基因表達及端粒酶活性等方面與hESC類似[85-86]。然而,從體細胞誘導成為iPSC過程中低效率、依賴于病毒介導的基因整合等一定程度上限制了iPSC的應用。對此,學者們提出了利用小分子化合物(如丙戊酸、鋰)、非整合型附加型載體、piggyBac轉座子、重組蛋白等多種方案[87-91]。與ESC類似,iPSC的分化方法也多種多樣,如基質細胞共培養、自發分化、重組蛋白與化合物誘導[7, 92]。至此,已有多項患者iPSC得以建立并成功分化為RPE細胞、光感受器細胞等,且被證實其在形態及電生理等功能上與同類細胞相似,并被應用于研究疾病機理及藥物篩選[93-97]。而臨床前試驗及臨床試驗的開展更是為干細胞臨床治療奠定了基礎。2012年,Li等[94]將iPSC誘導生成的RPE細胞注入出生2 d后的視網膜變性小鼠視網膜下腔內,在小鼠的整個生命周期中無腫瘤發生,且在長期觀察中ERG振幅有所提高。與之類似,iPSC誘導的光感受器細胞移植入視網膜變性早期及晚期小鼠及豬的視網膜下腔內,可觀察到移植的細胞整合進視網膜外核層,并生成似外節樣的投射樣形態。小鼠模型中b波振幅提高,光誘導的穿梭回避反應改善,且通過膜電極及多焦ERG分析證實RGC的電生理反應部分來自于植片[98-100]。以動物實驗為基礎,2017年AMD患者的iPSC建立,其誘導的RPE植片通過手術方式植入患者眼內后1年,雖無腫瘤生成,但患者BCVA也無明顯提高[101]。來源于患者的iPSC潛在問題值得重視,未經基因修正的iPSC,其分化出來的視網膜將出現同樣的疾病表型。干細胞移植前的基因修正必不可少。而CRISPR/Cas9技術的出現使基因修正更加簡便。Burnight等[102]利用此技術在iPSC上分別進行了對外顯子突變、剪接位點突變、顯性突變的基因修復,證明未出現編碼區域的基因脫靶。2018年Deng等[103]對RPGR基因突變的RP患者iPSC進行3D分化,觀察到纖毛縮短、異常的光感受器細胞形態、定位、轉錄譜及電生理活動,而經CRISPR/Cas9技術糾正基因突變后,其纖毛形態及基因表達再次得以恢復。這不僅模擬了RPGR基因突變引起的病理生理變化,也證明了CRISPR/Cas9技術在干細胞移植的應用前景。基于iPSC的干細胞治療勢不可擋,同時MSC的臨床試驗也在有序進行。前后有22例RP患者接受玻璃體腔MSC移植后無腫瘤發生;短期(3個月)視力相關的生活質量評估也顯示其生活質量得以提高[104-105]。
干細胞使21世紀再生醫學發生了翻天覆地的變化。與基因治療一樣,干細胞治療也在眼底病上實現了開創性勝利。然而,如何高效分化神經視網膜細胞?尤其是光感受器細胞,如何使移植神經元生存并發揮功能?還需要進一步研究。
3 展望
基因與干細胞治療運用為臨床治療遺傳性眼底病開拓了新道路。雖然面臨基因脫靶、分化效率、細胞遷移、長期療效問題等諸多問題[106-109],但其在臨床前期及臨床試驗中取得的成果不容小覷。而CRISPR/Cas9技術、納米材料等新技術和新材料的出現[110],勢必將進一步輔助基因與干細胞治療策略,為遺傳性眼底病的臨床治愈帶來無限機遇和無限可能。
| 1. | Sahel JA, Marazova K, Audo I. Clinical characteristics and current therapies for inherited retinal degenerations[J/OL]. Cold Spring Harb Perspect Med, 2014, 5(2): 017111[2014-10-16]. http://perspectivesinmedicine.cshlp.org/content/5/2/a017111.long. DOI: 10.1101/cshperspect.a017111. |
| 2. | Cideciyan AV, Sudharsan R, Dufour VL, et al. Mutation-independent rhodopsin gene therapy by knockdown and replacement with a single AAV vector[J]. Proc Natl Acad Sci USA, 2018, 115(36): 8547-8556. DOI: 10.1073/pnas.1805055115. |
| 3. | Ran X, Cai WJ, Huang XF, et al. RetinoGenetics: a comprehensive mutation database for genes related to inherited retinal degeneration[J/OL]. Database (Oxford), 2014, 2014: E1[2014-06- 17]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4060621/. DOI: 10.1093/database/bau047.[published online ahead of print]. |
| 4. | Trapani I, Banfi S, Simonelli F, et al. Gene therapy of Inherited retinal degenerations: prospects and challenges[J]. Hum Gene Ther, 2015.26: 193-200. DOI: 10.1089/hum.2015.030. |
| 5. | Musarella MA, Macdonald IM. Current concepts in the treatment of retinitis pigmentosa[J/OL]. J Ophthalmol, 2011, 2011: 753547[2010-10-11]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2964907/. DOI: 10.1155/2011/753547. |
| 6. | Petrs-Silva H, Linden R. Advances in gene therapy technologies to treat retinitis pigmentosa[J]. Clin Ophthalmol, 2014, 8: 127-136. DOI: 10.2147/OPTH.S38041. |
| 7. | 鄧雯麗, 向萍, 金子兵. 多能干細胞分化來源視網膜色素上皮細胞移植治療視網膜變性研究進展[J]. 中華細胞與干細胞雜志(電子版), 2014, 4(2): 97-103. DOI: 10.3877/cma.j.issn.2095-1221.2014.02.004.Deng WL, Xiang P, Jin ZB. The research progress toward clinical transplantation of pluripotent stem cell-derived retinal pigmented epithelial cells[J]. Chin J Cell Stem Cell (Electronic Edition), 2014, 4(2): 97-103. DOI: 10.3877/cma.j.issn.2095-1221.2014.02.004. |
| 8. | Bennicelli J, Wright JF, Komaromy A, et al. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer[J]. Mol Ther, 2008, 16(3): 458- 465. DOI: 10.1038/sj.mt.6300389. |
| 9. | Narfstr?m K, Katz ML, Bragadottir R, et al. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog[J]. Invest Ophthalmol Vis Sci, 2003, 44(4): 1663-1672. |
| 10. | Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness[J]. Nat Genet, 2001, 28(1): 92-95. DOI: 10.1038/88327. |
| 11. | Acland GM, Aguirre GD, Bennett J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness[J]. Mol Ther, 2005, 12(6):1072-1082. DOI: 10.1016/j.ymthe.2005.08.008. |
| 12. | Le Meur G, Stieger K, Smith AJ, et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium[J]. Gene Ther, 2007, 14(4): 292-303. DOI: 10.1038/sj.gt.3302861. |
| 13. | Pang JJ, Chang B, Kumar A, et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis[J]. Mol Ther, 2006, 13(3): 565-572. DOI: 10.1016/j.ymthe.2005.09.001. |
| 14. | Roman AJ, Boye SL, Aleman TS, et al. Electroretinographic analyses of RPE65-mutant rd12 mice: developing an in vivo bioassay for human gene therapy trials of Leber congenital amaurosis[J]. Mol Vis, 2007, 13: 1701-1710. |
| 15. | Chen Y, Moiseyev G, Takahashi Y, et al. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65-/- mice[J]. Invest Ophthalmol Vis Sci, 2006, 47(3): 1177-1184. DOI: 10.1167/iovs.05-0965. |
| 16. | Van Hooser JP, Liang Y, Maeda T, et al. Recovery of visual functions in a mouse model of Leber congenital amaurosis[J]. J Biol Chem, 2002, 277(21): 19173-19182. DOI: 10.1074/jbc.M112384200. |
| 17. | Jacobson SG, Acland GM, Aguirre GD et al. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection[J]. Mol Ther, 2006, 13(6): 1074-1084. DOI: 10.1016/j.ymthe.2006.03.005. |
| 18. | Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis[J]. N Engl J Med, 2008, 358(21): 2240-2248. DOI: 10.1056/NEJMoa0802315. |
| 19. | Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase Ⅰ trial[J]. Hum Gene Ther, 2008, 19(10): 979-990. DOI: 10.1089/hum.2008.107. |
| 20. | Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics[J]. Proc Nati Acad Sci USA, 2008, 105(39): 15112-15117. DOI: 10.1073/pnas.0807027105. |
| 21. | Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis[J]. N Engl J Med, 2008, 358(21): 2231-2239. DOI: 10.1056/NEJMoa0802268. |
| 22. | Bennett J, Wellman J, Marshall KA, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial[J]. Lancet, 2016, 388(10045): 661-672. DOI: 10.1016/S0140-6736(16)30371-3. |
| 23. | Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial[J]. Lancet, 2017, 390(10097): 849-860. DOI: 10.1016/S0140-6736(17)31868-8. |
| 24. | Koch S, Sothilingam V, Garcia Garrido M, et al. Gene therapy restores vision and delays degeneration in the CNGB1(-/-) mouse model of retinitis pigmentosa[J]. Hum Mol Genet, 2012, 21(20): 4486-4496. DOI: 10.1093/hmg/dds290. |
| 25. | Michalakis S, Koch S, Sothilingam V, et al. Gene therapy restores vision and delays degeneration in the CNGB1(-/-) mouse model of retinitis pigmentosa[J]. Adv Exp Med Biol, 2014, 801: 733-739. DOI: 10.1007/978-1-4614-3209-8_92. |
| 26. | Conlon TJ, Deng WT, Erger K, et al. Preclinical potency and safety studies of an AAV2-mediated gene therapy vector for the treatment of MERTK associated retinitis pigmentosa[J]. Hum Gene Ther Clin Dev, 2013, 24(1): 23-28. DOI: 10.1089/humc.2013.037. |
| 27. | Smith AJ, Scchlichtenbrede FC, Tschernutter M, et al. AAV-Mediated gene transfer slows photoreceptor loss in the RCS rat model of retinitis pigmentosa[J]. Mol Ther, 2003, 8(2): 188-195. |
| 28. | Petersen-Jones SM, Occelli LM, Winkler PA, et al. Patients and animal models of CNGβ1-deficient retinitis pigmentosa support gene augmentation approach[J]. J Clin Invest, 2018, 128(1): 190-206. DOI: 10.1172/JCI95161. |
| 29. | Beltran WA, Cideciyan AV, Iwabe S, et al. Successful arrest of photoreceptor and vision loss expands the therapeutic window of retinal gene therapy to later stages of disease[J]. Proc Natl Acad Sci USA, 2015, 112(43): 5844-5853. DOI: 10.1073/pnas.1509914112. |
| 30. | Pawlyk BS, Bulgakov OV, Sun X, et al. Photoreceptor rescue by an abbreviated human RPGR gene in a murine model of X-linked retinitis pigmentosa[J]. Gene Ther, 2016, 23(2): 196-204. DOI: 10.1038/gt.2015.93. |
| 31. | Beltran WA, Cideciyan AV, Boye SE, et al. Optimization of retinal gene therapy for X-linked retinitis pigmentosa due to RPGR mutations[J]. Mol Ther, 2017, 25(8): 1866-1880. DOI: 10.1016/j.ymthe.2017.05.004. |
| 32. | Beltran WA, Cideciyan AV, Lewin AS, et al. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa[J]. Proc Natl Acad Sci USA, 2012, 109(6):2132-2137. DOI: 10.1073/pnas.1118847109. |
| 33. | Tessitore A, Parisi F, Denti MA, et al. Preferential silencing of a common dominant rhodopsin mutation does not inhibit retinal degeneration in a transgenic model[J]. Mol Ther, 2006, 14(5): 692-699. DOI: 10.1016/j.ymthe.2006.07.008. |
| 34. | Bakondi B, Lv W, Lu B, et al. In vivo CRISPR/Cas9 gene editing corrects retinal dystrophy in the S334ter-3 rat model of autosomal dominant retinitis pigmentosa[J]. Mol Ther, 2016, 24(3): 556-563. DOI: 10.1038/mt.2015.220. |
| 35. | Latella MC, Di Salvo MT, Cocchiarella F, et al. In vivo editing of the human mutant rhodopsin gene by electroporation of plasmid-based CRISPR/Cas9 in the mouse retina[J]. Mol Ther Nucleic Acids, 2016, 5(11): 389. DOI: 10.1038/mtna.2016.92. |
| 36. | Jiang L, Zhang H, Dizhoor AM, et al. Long-term RNA interference gene therapy in a dominant retinitis pigmentosa mouse model[J]. Proc Natl Acad Sci USA, 2011, 108(45): 18476-18481. DOI: 10.1073/pnas.1112758108. |
| 37. | Gorbatyuk M, Justilien V, Liu J, et al. Suppression of mouse rhodopsin expression in vivo by AAV mediated siRNA delivery[J]. Vision Res, 2007, 47(9): 1202-1208. DOI: 10.1016/j.visres.2006.11.026. |
| 38. | O'Reilly M, Palfi A, Chadderton N, et al. RNA interference-mediated suppression and replacement of human rhodopsin in vivo[J]. Am J Hum Genet, 2007, 81(1): 127-135. DOI: 10.1086/519025. |
| 39. | Millington-Ward S, Chadderton N, O’Reilly M, et al. Suppression and replacement gene therapy for autosomal dominant disease in a murine model of dominant retinitis pigmentosa[J]. Mol Ther, 2011, 19(4): 642-649. DOI: 10.1038/mt.2010.293. |
| 40. | Mao H, Gorbatyuk MS, Rossmiller B, et al. Long-term rescue of retinal structure and function by rhodopsin RNA replacement with a single adeno-associated viral vector in P23H RHO transgenic mice[J]. Hum Gene Ther, 2012, 23(4): 356-366. DOI: 10.1089/hum.2011.213. |
| 41. | Chadderton N, Millington-Ward S, Palfi A, et al. Improved retinal function in a mouse model of dominant retinitis pigmentosa following AAV-delivered gene therapy[J]. Mol Ther, 2009, 17(4): 593-599. DOI: 10.1038/mt.2008.301. |
| 42. | Büning H, Perabo L, Coutelle O, et al. Recent developments in adeno-associated virus vector technology[J]. J Gene Med, 2008, 10(7): 717-733. DOI: 10.1002/jgm.1205. |
| 43. | Theodorou-Kanakari A, Karampitianis S, Karageorgou V, et al. Current and emerging treatment modalities for Leber's hereditary optic neuropathy: a review of the literature[J]. Adv Ther, 2018, 35(10): 1510-1518. DOI: 10.1007/s12325-018-0776-z. |
| 44. | MacLaren RE, Groppe M, Barnard AR, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial[J]. Lancet, 2014, 383(9923): 1129-1137. DOI: 10.1016/S0140-6736(13)62117-0. |
| 45. | Park TK, Wu Z, Kjellstrom S, et al. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse[J]. Gene Ther, 2009, 16(7): 916-926. DOI: 10.1038/gt.2009.61. |
| 46. | Buch PK, Bainbridge JW, Ali RR. AAV-mediated gene therapy for retinal disorders: from mouse to man[J]. Gene Ther, 2008, 15(11): 849-857. DOI: 10.1038/gt.2008.66. |
| 47. | Wu Z, Yang H, Colosi P. Effect of genome size on AAV vector packaging[J]. Mol Ther, 2010, 18(1):80-86. DOI: 10.1038/mt.2009.255. |
| 48. | Li Q, Miller R, Han PY, et al. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential[J]. Mol Vis, 2008, 14: 1760-1769. |
| 49. | Rajala A, Wang Y, Zhu Y, et al. Nanoparticle-assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo[J]. Nano Lett, 2014, 14(9): 5257- 5263. DOI: 10.1021/nl502275s. |
| 50. | Ochoa G, Sesma JZ, Díez MA, et al. A novel formulation based on 2, 3-di(tetradecyloxy) propan-1-amine cationic lipid combined with polysorbate 80 for efficient gene delivery to the retina[J]. Pharm Res, 2014, 31(7): 1665-1675. DOI: 10.1007/s11095-013-1271-5. |
| 51. | Koirala A, Conley SM, Naash MI. A review of therapeutic prospects of non-viral gene therapy in the retinal pigment epithelium[J]. Biomaterials, 2013, 34(29): 7158-7167. DOI: 10.1016/j.biomaterials.2013.06.002. |
| 52. | Bourges JL, Gautier SE, Delie F, et al. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles[J]. Invest Ophthalmol Vis Sci, 2003, 44(8): 3562-3569. |
| 53. | Koo H, Moon H, Han H, et al. The movement of self-assembled amphiphilic polymeric nanoparticles in the vitreous and retina after intravitreal injection[J]. Biomaterials, 2012, 33(12):3485-3493. DOI: 10.1016/j.biomaterials.2012.01.030. |
| 54. | Han Z, Banworth MJ, Makkia R, et al. Genomic DNA nanoparticles rescue rhodopsin-associated retinitis pigmentosa phenotype[J]. FASEB J, 2015, 29(6): 2535-2544. DOI: 10.1096/fj.15-270363. |
| 55. | Dalkara D, Kolstad KD, Caporale N, et al. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous[J]. Mol Ther, 2009, 17(12): 2096-2102. DOI: 10.1038/mt.2009.181. |
| 56. | Kolstad KD, Dalkara D, Guerin K, et al. Changes in adeno-associated virus-mediated gene delivery in retinal degeneration[J]. Hum Gene Ther, 2010, 21(5): 571-578. DOI: 10.1089/hum.2009.194. |
| 57. | Lewis GP, Charteris DG, Sethi CS, et al. Animal models of retinal detachment and reattachment: identifying cellular events that may affect visual recovery[J]. Eye (Lond), 2002, 16(4): 375-387. DOI: 10.1038/sj.eye.6700202. |
| 58. | Lewis GP, Sethi CS, Linberg KA, et al. Experimental retinal reattachment: a new perspective[J]. Mol Neurobiol, 2003, 28(2): 159-175. DOI: 10.1385/MN:28:2:159. |
| 59. | Fisher SK, Lewis GP. Müller cell and neuronal remodeling in retinal detachment and reattachment and their potential consequences for visual recovery: a review and reconsideration of recent data[J]. Vision Res, 2003, 43(8): 887-897. |
| 60. | Fisher SK, Lewis GP, Linberg KA, et al. Cellular remodeling in mammalian retina: results from studies of experimental retinal detachment[J]. Prog Retin Eye Res, 2005, 24(3): 395-431. DOI: 10.1016/j.preteyeres.2004.10.004. |
| 61. | Lebherz C, Maguire A, Tang W, et al. Novel AAV serotypes for improved ocular gene transfer[J]. J Gene Med, 2008, 10(4): 375-382. DOI: 10.1002/jgm.1126. |
| 62. | Harvey AR, Kamphuis W, Eggers R, et al. Intravitreal injection of adeno-associated viral vectors results in the transduction of different types of retinal neurons in neonatal and adult rats: a comparison with lentiviral vectors[J]. Mol Cell Neurosci, 2002, 21(1): 141-157. |
| 63. | Martin KR, Klein RL, Quigley HA. Gene delivery to the eye using adeno-associated viral vectors[J]. Methods, 2002, 28(2): 267-275. |
| 64. | Dalkara D, Byrne LC, Klimczak RR, et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous[J/OL]. Sci Trans Med, 2013, 5(189): 189ra76[2013-06-12]. http://stm.sciencemag.org/content/5/189/189ra76.long. DOI: 10.1126/scitranslmed.3005708. |
| 65. | Jayakody SA, Gonzalez-Cordero A, Ali RR, et al. Cellular strategies for retinal repair by photoreceptor replacement[J]. Prog Retin Eye Res, 2015, 46: 31-66. DOI: 10.1016/j.preteyeres.2015.01.003. |
| 66. | Mead B, Berry M, Logan A, et al. Stem cell treatment of degenerative eye disease[J]. Stem Cell Res, 2015, 14(3): 243-257. DOI: 10.1016/j.scr.2015.02.003. |
| 67. | Jin ZB, Gao ML, Deng WL, et al. Stemming retinal regeneration with pluripotent stem cells[J/OL]. Prog Retin Eye Res, 2018, 2018:E1[2018-11-09]. https://linkinghub.elsevier.com/retrieve/pii/S1350-9462(17)30123-4. DOI: 10.1016/j.preteyeres.2018.11.003. [published online ahead of print]. |
| 68. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts[J]. Science, 1998, 282(5391): 1145-1147. |
| 69. | Meyer JS, Katz ML, Maruniak JA, et al. Neural differentiation of mouse embryonic stem cells in vitro and after transplantation into eyes of mutant mice with rapid retinal degeneration[J]. Brain Res, 2004, 1014(1-2): 131-144. DOI: 10.1016/j.brainres.2004.04.019. |
| 70. | Meyer JS, Katz ML, Maruniak JA, et al. Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors[J]. Stem Cells, 2006, 24(2): 274-283. DOI: 10.1634/stemcells.2005-0059. |
| 71. | Banin E, Obolensky A, Idelson M, et al. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells[J]. Stem Cells, 2006, 24(2): 246-257. DOI: 10.1634/stemcells.2005-0009. |
| 72. | Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells[J]. Stem Cells, 2009, 27(10): 2427-2434. DOI: 10.1002/stem.189. |
| 73. | Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells[J]. Nat Biotechnol, 2008, 26(2): 215-224. DOI: 10.1038/nbt1384. |
| 74. | Assawachananont J, Mandai M, Okamoto S, et al. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice[J]. Stem Cell Reports, 2014, 2(5): 662-674. DOI: 10.1016/j.stemcr. |
| 75. | Shirai H, Mandai M, Matsushita K, et al. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration[J]. Proc Natl Acad Sci USA, 2016, 113(1): 81-90. DOI: 10.1073/pnas.1512590113. |
| 76. | Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells[J]. Cell Stem Cell, 2009, 5(4): 396-408. DOI: 10.1016/j.stem.2009.07.002. |
| 77. | Lu B, Malcuit C, Wang S, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration[J]. Stem Cells, 2009, 27(9): 2126-2135. DOI: 10.1002/stem.149. |
| 78. | Lund RD, Wang S, Klimanskaya I, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats[J]. Cloning Stem Cells, 2006, 8(3): 189-199. DOI: 10.1089/clo.2006.8.189. |
| 79. | Vugler A, Carr AJ, Lawrence J, et al. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation[J]. Exp Neurol, 2008, 214(2): 347-361. DOI: 10.1016/j.expneurol.2008.09.007. |
| 80. | Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report[J]. Lancet, 2012, 379(9817): 713-720. DOI: 10.1016/S0140-6736(12)60028-2. |
| 81. | Schwartz S, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies[J]. Lancet, 2015, 385(9967): 509-516. DOI: 10.1016/S0140-6736(14)61376-3. |
| 82. | Schwartz SD, Tan G, Hosseini H, et al. Subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium for the treatment of macular degeneration: an assessment at 4 years[J]. Invest Ophthalmol Vis Sci, 2016, 57(5): 1-9. DOI: 10.1167/iovs.15-18681. |
| 83. | Song WK, Park KM, Kim HJ, et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients[J]. Stem Cell Reports, 2015, 4(5): 860-872. DOI: 10.1016/j.stemcr.2015.04.005. |
| 84. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors[J]. Cell, 2006, 126(4): 663-676. DOI: 10.1016/j.cell.2006.07.024. |
| 85. | Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors[J]. Cell, 2007, 131(5): 861-872. |
| 86. | Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells[J]. Science, 2007, 318(5858): 1917-1920. DOI: 10.1126/science.1151526. |
| 87. | Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2[J]. Nat Biotechnol, 2008, 26(11): 1269-1275. DOI: 10.1038/nbt.1502. |
| 88. | Wang Q, Xu X, Li J, et al. Lithium, an anti-psychotic drug, greatly enhances the generation of induced pluripotent stem cells[J]. Cell Res, 2011, 21(10): 1424-1435. DOI: 10.1038/cr.2011.108. |
| 89. | Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences[J]. Science, 2009, 324(5928): 797-801. DOI: 10.1126/science.1172482. |
| 90. | Woltjen K, Michael IP, Mohseni P, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells[J]. Nature, 2009, 458(7239): 766-770. DOI: 10.1038/nature07863. |
| 91. | Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins[J]. Cell Stem Cell, 2009, 4(6): 472-476. DOI: 10.1016/j.stem.2009.05.005. |
| 92. | Osakada F, Jin ZB, Hirami Y, et al. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction[J]. J Cell Sci, 2009, 122(Pt 17): 3169-3179. DOI: 10.1242/jcs.050393. |
| 93. | Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells[J]. Cell, 2008, 134(5): 877-886. DOI: 10.1016/j.cell.2008.07.041. |
| 94. | Li Y, Tsai YT, Hsu CW, et al. Long-term safety and efficacy of human-induced pluripotent stem cell (iPS) grafts in a preclinical model of retinitis pigmentosa[J]. Mol Med, 2012, 18: 1312- 1319. DOI: 10.2119/molmed.2012.00242. |
| 95. | Lamba DA, McUsic A, Hirata RK, et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells[J/OL]. PLoS One, 2010, 5(1): 8763[2010-01-20]. https://doi.org/10.1371/journal.pone.0008763. DOI: 10.1371/journal.pone.0008763. |
| 96. | Jin ZB, Okamoto S, Osakada F, et al. Modeling retinal degeneration using patient-specific induced pluripotent stem cells[J/OL]. PLoS One, 2011, 6(2): 17084[2011-02-10]. https://doi.org/10.1371/journal.pone.0017084. DOI: 10.1371/journal.pone.0017084. |
| 97. | Jin ZB, Okamoto S, Xiang P, et al. Integration-free induced pluripotent stem cells derived from retinitis pigmentosa patient for disease modeling[J]. Stem Cells Transl Med, 2012, 1(6): 503- 509. DOI: 10.5966/sctm.2012-0005. |
| 98. | Tucker BA, Park IH, Qi SD, et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice[J/OL]. PLoS One, 2011, 6(4): 18992[2011-04-29]. https://doi.org/10.1371/journal.pone.0018992. DOI: 10.1371/journal.pone.0018992. |
| 99. | Zhou L, Wang W, Liu Y, et al. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina[J]. Stem Cells, 2011, 29(6): 972-980. DOI: 10.1002/stem.637. |
| 100. | Mandai M, Fujii M, Hashiguchi T, et al. iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice[J]. Stem Cell Reports, 2017, 8(1): 69-83. DOI: 10.1016/j.stemcr.2016.12.008. |
| 101. | Mandai M, Watanabe A, Kurimoto Y, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration[J]. N Engl J Med, 2017, 376(11): 1038-1046. DOI: 10.1056/NEJMoa1608368. |
| 102. | Burnight ER, Gupta M, Wiley LA, et al. Using CRISPR-Cas9 to generate gene-corrected autologous iPSCs for the treatment of inherited retinal degeneration[J]. Mol Ther, 2017, 25(9): 1999-2013. DOI: 10.1016/j.ymthe.2017.05.015. |
| 103. | Deng WL, Gao ML, Lei XL, et al. Gene correction reverses ciliopathy and photoreceptor loss in iPSC-derived retinal organoids from retinitis pigmentosa patients[J]. Stem Cell Reports, 2018, 10(4): 1267-1281. DOI: 10.1016/j.stemcr.2018.02.003. |
| 104. | Siqueira RC, Messias A, Messias K, et al. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell-clinical trial)[J]. Stem Cell Res Ther, 2015, 6: 29. DOI: 10.1186/s13287-015-0020-6. |
| 105. | Siqueira RC, Messias A, Voltarelli JC, et al. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a phase Ⅰtrial[J]. Retina, 2011, 31(6): 1207-1214. DOI: 10.1097/IAE.0b013e3181f9c242. |
| 106. | Kuriyan AE, Albini TA, Townsend JH, et al. Vision loss after intravitreal injection of autologous "stem cells" for AMD[J]. N Engl J Med, 2017, 376(11): 1047-1053. DOI: 10.1056/NEJMoa1609583. |
| 107. | Wang NK, Tosi J, Kasanuki JM, et al. Transplantation of reprogrammed embryonic stem cells improves visual function in a mouse model for retinitis pigmentosa[J]. Transplantation, 2016, 89(8): 911-919. DOI: 10.1097/TP.0b013e3181d45a61. |
| 108. | Jacobson SG, Cideciyan AV, RomanAJ, et al. Improvement and decline in vision with gene therapy in childhood blindness[J]. N Engl J Med, 2015, 372(20): 1920-1926. DOI: 10.1056/NEJMoa1412965. |
| 109. | Bainbridge JW, Mehat MS, Sundaram V, et al. Long-term effect of gene therapy on Leber's congenital amaurosis[J]. N Engl J Med, 2015, 372(20): 1887-1897. DOI: 10.1056/NEJMoa1414221. |
| 110. | Burnight ER, Giacalone JC, Cooke JA, et al. CRISPR-Cas9 genome engineering: Treating inherited retinal degeneration[J]. Prog Retin Eye Res, 2018, 65: 28-49. DOI: 10.1016/j.preteyeres.2018.03.003. |
- 1. Sahel JA, Marazova K, Audo I. Clinical characteristics and current therapies for inherited retinal degenerations[J/OL]. Cold Spring Harb Perspect Med, 2014, 5(2): 017111[2014-10-16]. http://perspectivesinmedicine.cshlp.org/content/5/2/a017111.long. DOI: 10.1101/cshperspect.a017111.
- 2. Cideciyan AV, Sudharsan R, Dufour VL, et al. Mutation-independent rhodopsin gene therapy by knockdown and replacement with a single AAV vector[J]. Proc Natl Acad Sci USA, 2018, 115(36): 8547-8556. DOI: 10.1073/pnas.1805055115.
- 3. Ran X, Cai WJ, Huang XF, et al. RetinoGenetics: a comprehensive mutation database for genes related to inherited retinal degeneration[J/OL]. Database (Oxford), 2014, 2014: E1[2014-06- 17]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4060621/. DOI: 10.1093/database/bau047.[published online ahead of print].
- 4. Trapani I, Banfi S, Simonelli F, et al. Gene therapy of Inherited retinal degenerations: prospects and challenges[J]. Hum Gene Ther, 2015.26: 193-200. DOI: 10.1089/hum.2015.030.
- 5. Musarella MA, Macdonald IM. Current concepts in the treatment of retinitis pigmentosa[J/OL]. J Ophthalmol, 2011, 2011: 753547[2010-10-11]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2964907/. DOI: 10.1155/2011/753547.
- 6. Petrs-Silva H, Linden R. Advances in gene therapy technologies to treat retinitis pigmentosa[J]. Clin Ophthalmol, 2014, 8: 127-136. DOI: 10.2147/OPTH.S38041.
- 7. 鄧雯麗, 向萍, 金子兵. 多能干細胞分化來源視網膜色素上皮細胞移植治療視網膜變性研究進展[J]. 中華細胞與干細胞雜志(電子版), 2014, 4(2): 97-103. DOI: 10.3877/cma.j.issn.2095-1221.2014.02.004.Deng WL, Xiang P, Jin ZB. The research progress toward clinical transplantation of pluripotent stem cell-derived retinal pigmented epithelial cells[J]. Chin J Cell Stem Cell (Electronic Edition), 2014, 4(2): 97-103. DOI: 10.3877/cma.j.issn.2095-1221.2014.02.004.
- 8. Bennicelli J, Wright JF, Komaromy A, et al. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer[J]. Mol Ther, 2008, 16(3): 458- 465. DOI: 10.1038/sj.mt.6300389.
- 9. Narfstr?m K, Katz ML, Bragadottir R, et al. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog[J]. Invest Ophthalmol Vis Sci, 2003, 44(4): 1663-1672.
- 10. Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness[J]. Nat Genet, 2001, 28(1): 92-95. DOI: 10.1038/88327.
- 11. Acland GM, Aguirre GD, Bennett J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness[J]. Mol Ther, 2005, 12(6):1072-1082. DOI: 10.1016/j.ymthe.2005.08.008.
- 12. Le Meur G, Stieger K, Smith AJ, et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium[J]. Gene Ther, 2007, 14(4): 292-303. DOI: 10.1038/sj.gt.3302861.
- 13. Pang JJ, Chang B, Kumar A, et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis[J]. Mol Ther, 2006, 13(3): 565-572. DOI: 10.1016/j.ymthe.2005.09.001.
- 14. Roman AJ, Boye SL, Aleman TS, et al. Electroretinographic analyses of RPE65-mutant rd12 mice: developing an in vivo bioassay for human gene therapy trials of Leber congenital amaurosis[J]. Mol Vis, 2007, 13: 1701-1710.
- 15. Chen Y, Moiseyev G, Takahashi Y, et al. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65-/- mice[J]. Invest Ophthalmol Vis Sci, 2006, 47(3): 1177-1184. DOI: 10.1167/iovs.05-0965.
- 16. Van Hooser JP, Liang Y, Maeda T, et al. Recovery of visual functions in a mouse model of Leber congenital amaurosis[J]. J Biol Chem, 2002, 277(21): 19173-19182. DOI: 10.1074/jbc.M112384200.
- 17. Jacobson SG, Acland GM, Aguirre GD et al. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection[J]. Mol Ther, 2006, 13(6): 1074-1084. DOI: 10.1016/j.ymthe.2006.03.005.
- 18. Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis[J]. N Engl J Med, 2008, 358(21): 2240-2248. DOI: 10.1056/NEJMoa0802315.
- 19. Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase Ⅰ trial[J]. Hum Gene Ther, 2008, 19(10): 979-990. DOI: 10.1089/hum.2008.107.
- 20. Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics[J]. Proc Nati Acad Sci USA, 2008, 105(39): 15112-15117. DOI: 10.1073/pnas.0807027105.
- 21. Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis[J]. N Engl J Med, 2008, 358(21): 2231-2239. DOI: 10.1056/NEJMoa0802268.
- 22. Bennett J, Wellman J, Marshall KA, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial[J]. Lancet, 2016, 388(10045): 661-672. DOI: 10.1016/S0140-6736(16)30371-3.
- 23. Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial[J]. Lancet, 2017, 390(10097): 849-860. DOI: 10.1016/S0140-6736(17)31868-8.
- 24. Koch S, Sothilingam V, Garcia Garrido M, et al. Gene therapy restores vision and delays degeneration in the CNGB1(-/-) mouse model of retinitis pigmentosa[J]. Hum Mol Genet, 2012, 21(20): 4486-4496. DOI: 10.1093/hmg/dds290.
- 25. Michalakis S, Koch S, Sothilingam V, et al. Gene therapy restores vision and delays degeneration in the CNGB1(-/-) mouse model of retinitis pigmentosa[J]. Adv Exp Med Biol, 2014, 801: 733-739. DOI: 10.1007/978-1-4614-3209-8_92.
- 26. Conlon TJ, Deng WT, Erger K, et al. Preclinical potency and safety studies of an AAV2-mediated gene therapy vector for the treatment of MERTK associated retinitis pigmentosa[J]. Hum Gene Ther Clin Dev, 2013, 24(1): 23-28. DOI: 10.1089/humc.2013.037.
- 27. Smith AJ, Scchlichtenbrede FC, Tschernutter M, et al. AAV-Mediated gene transfer slows photoreceptor loss in the RCS rat model of retinitis pigmentosa[J]. Mol Ther, 2003, 8(2): 188-195.
- 28. Petersen-Jones SM, Occelli LM, Winkler PA, et al. Patients and animal models of CNGβ1-deficient retinitis pigmentosa support gene augmentation approach[J]. J Clin Invest, 2018, 128(1): 190-206. DOI: 10.1172/JCI95161.
- 29. Beltran WA, Cideciyan AV, Iwabe S, et al. Successful arrest of photoreceptor and vision loss expands the therapeutic window of retinal gene therapy to later stages of disease[J]. Proc Natl Acad Sci USA, 2015, 112(43): 5844-5853. DOI: 10.1073/pnas.1509914112.
- 30. Pawlyk BS, Bulgakov OV, Sun X, et al. Photoreceptor rescue by an abbreviated human RPGR gene in a murine model of X-linked retinitis pigmentosa[J]. Gene Ther, 2016, 23(2): 196-204. DOI: 10.1038/gt.2015.93.
- 31. Beltran WA, Cideciyan AV, Boye SE, et al. Optimization of retinal gene therapy for X-linked retinitis pigmentosa due to RPGR mutations[J]. Mol Ther, 2017, 25(8): 1866-1880. DOI: 10.1016/j.ymthe.2017.05.004.
- 32. Beltran WA, Cideciyan AV, Lewin AS, et al. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa[J]. Proc Natl Acad Sci USA, 2012, 109(6):2132-2137. DOI: 10.1073/pnas.1118847109.
- 33. Tessitore A, Parisi F, Denti MA, et al. Preferential silencing of a common dominant rhodopsin mutation does not inhibit retinal degeneration in a transgenic model[J]. Mol Ther, 2006, 14(5): 692-699. DOI: 10.1016/j.ymthe.2006.07.008.
- 34. Bakondi B, Lv W, Lu B, et al. In vivo CRISPR/Cas9 gene editing corrects retinal dystrophy in the S334ter-3 rat model of autosomal dominant retinitis pigmentosa[J]. Mol Ther, 2016, 24(3): 556-563. DOI: 10.1038/mt.2015.220.
- 35. Latella MC, Di Salvo MT, Cocchiarella F, et al. In vivo editing of the human mutant rhodopsin gene by electroporation of plasmid-based CRISPR/Cas9 in the mouse retina[J]. Mol Ther Nucleic Acids, 2016, 5(11): 389. DOI: 10.1038/mtna.2016.92.
- 36. Jiang L, Zhang H, Dizhoor AM, et al. Long-term RNA interference gene therapy in a dominant retinitis pigmentosa mouse model[J]. Proc Natl Acad Sci USA, 2011, 108(45): 18476-18481. DOI: 10.1073/pnas.1112758108.
- 37. Gorbatyuk M, Justilien V, Liu J, et al. Suppression of mouse rhodopsin expression in vivo by AAV mediated siRNA delivery[J]. Vision Res, 2007, 47(9): 1202-1208. DOI: 10.1016/j.visres.2006.11.026.
- 38. O'Reilly M, Palfi A, Chadderton N, et al. RNA interference-mediated suppression and replacement of human rhodopsin in vivo[J]. Am J Hum Genet, 2007, 81(1): 127-135. DOI: 10.1086/519025.
- 39. Millington-Ward S, Chadderton N, O’Reilly M, et al. Suppression and replacement gene therapy for autosomal dominant disease in a murine model of dominant retinitis pigmentosa[J]. Mol Ther, 2011, 19(4): 642-649. DOI: 10.1038/mt.2010.293.
- 40. Mao H, Gorbatyuk MS, Rossmiller B, et al. Long-term rescue of retinal structure and function by rhodopsin RNA replacement with a single adeno-associated viral vector in P23H RHO transgenic mice[J]. Hum Gene Ther, 2012, 23(4): 356-366. DOI: 10.1089/hum.2011.213.
- 41. Chadderton N, Millington-Ward S, Palfi A, et al. Improved retinal function in a mouse model of dominant retinitis pigmentosa following AAV-delivered gene therapy[J]. Mol Ther, 2009, 17(4): 593-599. DOI: 10.1038/mt.2008.301.
- 42. Büning H, Perabo L, Coutelle O, et al. Recent developments in adeno-associated virus vector technology[J]. J Gene Med, 2008, 10(7): 717-733. DOI: 10.1002/jgm.1205.
- 43. Theodorou-Kanakari A, Karampitianis S, Karageorgou V, et al. Current and emerging treatment modalities for Leber's hereditary optic neuropathy: a review of the literature[J]. Adv Ther, 2018, 35(10): 1510-1518. DOI: 10.1007/s12325-018-0776-z.
- 44. MacLaren RE, Groppe M, Barnard AR, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial[J]. Lancet, 2014, 383(9923): 1129-1137. DOI: 10.1016/S0140-6736(13)62117-0.
- 45. Park TK, Wu Z, Kjellstrom S, et al. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse[J]. Gene Ther, 2009, 16(7): 916-926. DOI: 10.1038/gt.2009.61.
- 46. Buch PK, Bainbridge JW, Ali RR. AAV-mediated gene therapy for retinal disorders: from mouse to man[J]. Gene Ther, 2008, 15(11): 849-857. DOI: 10.1038/gt.2008.66.
- 47. Wu Z, Yang H, Colosi P. Effect of genome size on AAV vector packaging[J]. Mol Ther, 2010, 18(1):80-86. DOI: 10.1038/mt.2009.255.
- 48. Li Q, Miller R, Han PY, et al. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential[J]. Mol Vis, 2008, 14: 1760-1769.
- 49. Rajala A, Wang Y, Zhu Y, et al. Nanoparticle-assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo[J]. Nano Lett, 2014, 14(9): 5257- 5263. DOI: 10.1021/nl502275s.
- 50. Ochoa G, Sesma JZ, Díez MA, et al. A novel formulation based on 2, 3-di(tetradecyloxy) propan-1-amine cationic lipid combined with polysorbate 80 for efficient gene delivery to the retina[J]. Pharm Res, 2014, 31(7): 1665-1675. DOI: 10.1007/s11095-013-1271-5.
- 51. Koirala A, Conley SM, Naash MI. A review of therapeutic prospects of non-viral gene therapy in the retinal pigment epithelium[J]. Biomaterials, 2013, 34(29): 7158-7167. DOI: 10.1016/j.biomaterials.2013.06.002.
- 52. Bourges JL, Gautier SE, Delie F, et al. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles[J]. Invest Ophthalmol Vis Sci, 2003, 44(8): 3562-3569.
- 53. Koo H, Moon H, Han H, et al. The movement of self-assembled amphiphilic polymeric nanoparticles in the vitreous and retina after intravitreal injection[J]. Biomaterials, 2012, 33(12):3485-3493. DOI: 10.1016/j.biomaterials.2012.01.030.
- 54. Han Z, Banworth MJ, Makkia R, et al. Genomic DNA nanoparticles rescue rhodopsin-associated retinitis pigmentosa phenotype[J]. FASEB J, 2015, 29(6): 2535-2544. DOI: 10.1096/fj.15-270363.
- 55. Dalkara D, Kolstad KD, Caporale N, et al. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous[J]. Mol Ther, 2009, 17(12): 2096-2102. DOI: 10.1038/mt.2009.181.
- 56. Kolstad KD, Dalkara D, Guerin K, et al. Changes in adeno-associated virus-mediated gene delivery in retinal degeneration[J]. Hum Gene Ther, 2010, 21(5): 571-578. DOI: 10.1089/hum.2009.194.
- 57. Lewis GP, Charteris DG, Sethi CS, et al. Animal models of retinal detachment and reattachment: identifying cellular events that may affect visual recovery[J]. Eye (Lond), 2002, 16(4): 375-387. DOI: 10.1038/sj.eye.6700202.
- 58. Lewis GP, Sethi CS, Linberg KA, et al. Experimental retinal reattachment: a new perspective[J]. Mol Neurobiol, 2003, 28(2): 159-175. DOI: 10.1385/MN:28:2:159.
- 59. Fisher SK, Lewis GP. Müller cell and neuronal remodeling in retinal detachment and reattachment and their potential consequences for visual recovery: a review and reconsideration of recent data[J]. Vision Res, 2003, 43(8): 887-897.
- 60. Fisher SK, Lewis GP, Linberg KA, et al. Cellular remodeling in mammalian retina: results from studies of experimental retinal detachment[J]. Prog Retin Eye Res, 2005, 24(3): 395-431. DOI: 10.1016/j.preteyeres.2004.10.004.
- 61. Lebherz C, Maguire A, Tang W, et al. Novel AAV serotypes for improved ocular gene transfer[J]. J Gene Med, 2008, 10(4): 375-382. DOI: 10.1002/jgm.1126.
- 62. Harvey AR, Kamphuis W, Eggers R, et al. Intravitreal injection of adeno-associated viral vectors results in the transduction of different types of retinal neurons in neonatal and adult rats: a comparison with lentiviral vectors[J]. Mol Cell Neurosci, 2002, 21(1): 141-157.
- 63. Martin KR, Klein RL, Quigley HA. Gene delivery to the eye using adeno-associated viral vectors[J]. Methods, 2002, 28(2): 267-275.
- 64. Dalkara D, Byrne LC, Klimczak RR, et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous[J/OL]. Sci Trans Med, 2013, 5(189): 189ra76[2013-06-12]. http://stm.sciencemag.org/content/5/189/189ra76.long. DOI: 10.1126/scitranslmed.3005708.
- 65. Jayakody SA, Gonzalez-Cordero A, Ali RR, et al. Cellular strategies for retinal repair by photoreceptor replacement[J]. Prog Retin Eye Res, 2015, 46: 31-66. DOI: 10.1016/j.preteyeres.2015.01.003.
- 66. Mead B, Berry M, Logan A, et al. Stem cell treatment of degenerative eye disease[J]. Stem Cell Res, 2015, 14(3): 243-257. DOI: 10.1016/j.scr.2015.02.003.
- 67. Jin ZB, Gao ML, Deng WL, et al. Stemming retinal regeneration with pluripotent stem cells[J/OL]. Prog Retin Eye Res, 2018, 2018:E1[2018-11-09]. https://linkinghub.elsevier.com/retrieve/pii/S1350-9462(17)30123-4. DOI: 10.1016/j.preteyeres.2018.11.003. [published online ahead of print].
- 68. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts[J]. Science, 1998, 282(5391): 1145-1147.
- 69. Meyer JS, Katz ML, Maruniak JA, et al. Neural differentiation of mouse embryonic stem cells in vitro and after transplantation into eyes of mutant mice with rapid retinal degeneration[J]. Brain Res, 2004, 1014(1-2): 131-144. DOI: 10.1016/j.brainres.2004.04.019.
- 70. Meyer JS, Katz ML, Maruniak JA, et al. Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors[J]. Stem Cells, 2006, 24(2): 274-283. DOI: 10.1634/stemcells.2005-0059.
- 71. Banin E, Obolensky A, Idelson M, et al. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells[J]. Stem Cells, 2006, 24(2): 246-257. DOI: 10.1634/stemcells.2005-0009.
- 72. Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells[J]. Stem Cells, 2009, 27(10): 2427-2434. DOI: 10.1002/stem.189.
- 73. Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells[J]. Nat Biotechnol, 2008, 26(2): 215-224. DOI: 10.1038/nbt1384.
- 74. Assawachananont J, Mandai M, Okamoto S, et al. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice[J]. Stem Cell Reports, 2014, 2(5): 662-674. DOI: 10.1016/j.stemcr.
- 75. Shirai H, Mandai M, Matsushita K, et al. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration[J]. Proc Natl Acad Sci USA, 2016, 113(1): 81-90. DOI: 10.1073/pnas.1512590113.
- 76. Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells[J]. Cell Stem Cell, 2009, 5(4): 396-408. DOI: 10.1016/j.stem.2009.07.002.
- 77. Lu B, Malcuit C, Wang S, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration[J]. Stem Cells, 2009, 27(9): 2126-2135. DOI: 10.1002/stem.149.
- 78. Lund RD, Wang S, Klimanskaya I, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats[J]. Cloning Stem Cells, 2006, 8(3): 189-199. DOI: 10.1089/clo.2006.8.189.
- 79. Vugler A, Carr AJ, Lawrence J, et al. Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation[J]. Exp Neurol, 2008, 214(2): 347-361. DOI: 10.1016/j.expneurol.2008.09.007.
- 80. Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report[J]. Lancet, 2012, 379(9817): 713-720. DOI: 10.1016/S0140-6736(12)60028-2.
- 81. Schwartz S, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies[J]. Lancet, 2015, 385(9967): 509-516. DOI: 10.1016/S0140-6736(14)61376-3.
- 82. Schwartz SD, Tan G, Hosseini H, et al. Subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium for the treatment of macular degeneration: an assessment at 4 years[J]. Invest Ophthalmol Vis Sci, 2016, 57(5): 1-9. DOI: 10.1167/iovs.15-18681.
- 83. Song WK, Park KM, Kim HJ, et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: preliminary results in Asian patients[J]. Stem Cell Reports, 2015, 4(5): 860-872. DOI: 10.1016/j.stemcr.2015.04.005.
- 84. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors[J]. Cell, 2006, 126(4): 663-676. DOI: 10.1016/j.cell.2006.07.024.
- 85. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors[J]. Cell, 2007, 131(5): 861-872.
- 86. Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells[J]. Science, 2007, 318(5858): 1917-1920. DOI: 10.1126/science.1151526.
- 87. Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2[J]. Nat Biotechnol, 2008, 26(11): 1269-1275. DOI: 10.1038/nbt.1502.
- 88. Wang Q, Xu X, Li J, et al. Lithium, an anti-psychotic drug, greatly enhances the generation of induced pluripotent stem cells[J]. Cell Res, 2011, 21(10): 1424-1435. DOI: 10.1038/cr.2011.108.
- 89. Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences[J]. Science, 2009, 324(5928): 797-801. DOI: 10.1126/science.1172482.
- 90. Woltjen K, Michael IP, Mohseni P, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells[J]. Nature, 2009, 458(7239): 766-770. DOI: 10.1038/nature07863.
- 91. Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins[J]. Cell Stem Cell, 2009, 4(6): 472-476. DOI: 10.1016/j.stem.2009.05.005.
- 92. Osakada F, Jin ZB, Hirami Y, et al. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction[J]. J Cell Sci, 2009, 122(Pt 17): 3169-3179. DOI: 10.1242/jcs.050393.
- 93. Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells[J]. Cell, 2008, 134(5): 877-886. DOI: 10.1016/j.cell.2008.07.041.
- 94. Li Y, Tsai YT, Hsu CW, et al. Long-term safety and efficacy of human-induced pluripotent stem cell (iPS) grafts in a preclinical model of retinitis pigmentosa[J]. Mol Med, 2012, 18: 1312- 1319. DOI: 10.2119/molmed.2012.00242.
- 95. Lamba DA, McUsic A, Hirata RK, et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells[J/OL]. PLoS One, 2010, 5(1): 8763[2010-01-20]. https://doi.org/10.1371/journal.pone.0008763. DOI: 10.1371/journal.pone.0008763.
- 96. Jin ZB, Okamoto S, Osakada F, et al. Modeling retinal degeneration using patient-specific induced pluripotent stem cells[J/OL]. PLoS One, 2011, 6(2): 17084[2011-02-10]. https://doi.org/10.1371/journal.pone.0017084. DOI: 10.1371/journal.pone.0017084.
- 97. Jin ZB, Okamoto S, Xiang P, et al. Integration-free induced pluripotent stem cells derived from retinitis pigmentosa patient for disease modeling[J]. Stem Cells Transl Med, 2012, 1(6): 503- 509. DOI: 10.5966/sctm.2012-0005.
- 98. Tucker BA, Park IH, Qi SD, et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice[J/OL]. PLoS One, 2011, 6(4): 18992[2011-04-29]. https://doi.org/10.1371/journal.pone.0018992. DOI: 10.1371/journal.pone.0018992.
- 99. Zhou L, Wang W, Liu Y, et al. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina[J]. Stem Cells, 2011, 29(6): 972-980. DOI: 10.1002/stem.637.
- 100. Mandai M, Fujii M, Hashiguchi T, et al. iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice[J]. Stem Cell Reports, 2017, 8(1): 69-83. DOI: 10.1016/j.stemcr.2016.12.008.
- 101. Mandai M, Watanabe A, Kurimoto Y, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration[J]. N Engl J Med, 2017, 376(11): 1038-1046. DOI: 10.1056/NEJMoa1608368.
- 102. Burnight ER, Gupta M, Wiley LA, et al. Using CRISPR-Cas9 to generate gene-corrected autologous iPSCs for the treatment of inherited retinal degeneration[J]. Mol Ther, 2017, 25(9): 1999-2013. DOI: 10.1016/j.ymthe.2017.05.015.
- 103. Deng WL, Gao ML, Lei XL, et al. Gene correction reverses ciliopathy and photoreceptor loss in iPSC-derived retinal organoids from retinitis pigmentosa patients[J]. Stem Cell Reports, 2018, 10(4): 1267-1281. DOI: 10.1016/j.stemcr.2018.02.003.
- 104. Siqueira RC, Messias A, Messias K, et al. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell-clinical trial)[J]. Stem Cell Res Ther, 2015, 6: 29. DOI: 10.1186/s13287-015-0020-6.
- 105. Siqueira RC, Messias A, Voltarelli JC, et al. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a phase Ⅰtrial[J]. Retina, 2011, 31(6): 1207-1214. DOI: 10.1097/IAE.0b013e3181f9c242.
- 106. Kuriyan AE, Albini TA, Townsend JH, et al. Vision loss after intravitreal injection of autologous "stem cells" for AMD[J]. N Engl J Med, 2017, 376(11): 1047-1053. DOI: 10.1056/NEJMoa1609583.
- 107. Wang NK, Tosi J, Kasanuki JM, et al. Transplantation of reprogrammed embryonic stem cells improves visual function in a mouse model for retinitis pigmentosa[J]. Transplantation, 2016, 89(8): 911-919. DOI: 10.1097/TP.0b013e3181d45a61.
- 108. Jacobson SG, Cideciyan AV, RomanAJ, et al. Improvement and decline in vision with gene therapy in childhood blindness[J]. N Engl J Med, 2015, 372(20): 1920-1926. DOI: 10.1056/NEJMoa1412965.
- 109. Bainbridge JW, Mehat MS, Sundaram V, et al. Long-term effect of gene therapy on Leber's congenital amaurosis[J]. N Engl J Med, 2015, 372(20): 1887-1897. DOI: 10.1056/NEJMoa1414221.
- 110. Burnight ER, Giacalone JC, Cooke JA, et al. CRISPR-Cas9 genome engineering: Treating inherited retinal degeneration[J]. Prog Retin Eye Res, 2018, 65: 28-49. DOI: 10.1016/j.preteyeres.2018.03.003.