肝細胞癌死亡信號蛋白TIFA抑癌新機制
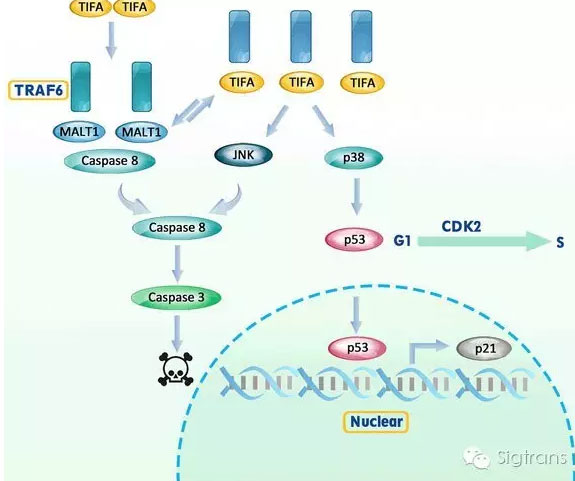
在全球范圍內,肝癌是癌癥死亡的第三大原因,南開大學的向榮教授和羅娜教授領導的團隊通過對肝細胞癌(HCC)患者進行了肝活檢以及小鼠皮下接種肝癌細胞等研究,在肝癌治療上取得了最新進展。他們的研究發現,肝細胞癌死亡信號蛋白TIFA蛋白可以通過三種途徑來抑制肝細胞癌的進展。其中之一是MALT1相關通路, TIFA與MALT1競爭結合TRAF6蛋白,這條信號通路會誘導癌細胞死亡。除此之外,TIFA的表達也會激活兩個基因,JNK和p38,這兩者分別與細胞死亡和細胞周期阻滯相關。該研究讓我們對這個影響HCC進展的藥物靶點有了更深入的認識。
Liver cancer: Protein signals cancer cell death
Research elucidating how a protein suppresses the progression of liver cancer could provide new therapeutic targets, say researchers in China. A team led by Rong Xiang and Na Luo at Nankai University examined liver biopsies of patients with hepatocellular carcinoma (HCC), the third most common cause of cancer-related death worldwide. They also grafted HCC cells under the skin of mice. They found that a protein, called TIFA, suppresses the progression of HCC via one of three pathways. One involves competition with another protein, MALT1, to bind with the protein TRAF6, signaling pathways that induce cancer cell death. TIFA-induced cell death also results from suppressing MALT1. Finally, TIFA expression can also activate two genes, JNK and p38, which signal cell death and cell cycle arrest, respectively. The research may provide insights into drug targets that could affect HCC progression.
TIFA, also called T2BP, was first identified using yeast two-hybrid screening. Our previous work showed that TIFA suppresses hepatocellular carcinoma (HCC) progression via apoptosis and cell cycle arrest. However, the mechanism by which this TIFA suppression occurs remains unclear. Here we demonstrated that TIFA-induced apoptosis demonstrates two distinct time patterns (i.e., at 48?h and >7 days) when TIFA reconstitution occurs. Moreover, we found that MALT1 (a competitor of TIFA) plays a crucial role in short-duration TIFA reconstitution. In this regard, MALT1 silencing with shRNA markedly enhances TIFA-induced apoptosis in vitro and in vivo. In addition, TIFA overexpression triggers JNK and p38 activation in long-duration TIFA reconstitution through TRAF6 binding. In particular, JNK activation leads to TIFA-induced apoptosis while p38 activation governs TIFA-induced cell cycle arrest by p53-p21 signaling in vitro and in vivo. Our data suggest a novel mechanism by which TIFA suppresses HCC progression via both MALT1-dependent and MALT1-independent signaling pathways. This may provide insights into a novel targets where HCC progression may be vulnerable to clinical treatment.
Qingxiang Sun, Xueqin Chen, Qiao Zhou, Ezra Burstein, Shengyong Yang, Da Jia. Inhibiting cancer cell hallmark features through nuclear export inhibition. Signal transduction and targeted therapy, 1. (2016). doi:10.1038/sigtrans.2016.10