2016-06-24 Steven Rokita Sigtrans
編輯總結(Editorial Summary)
藥物研發:更精準的化療
能可逆轉結合并修改特定的DNA序列的化合物為更安全的癌癥治療提供了基礎。目前,許多化療藥物可以通過在基因組的任一位點引入不可逆轉的改變來殺死癌癥細胞,這導致這些化療藥物同樣可以損害非目標細胞,同時也消耗大量的抗癌藥物。馬里蘭大學的Steven Rokita教授與其在中國的合作伙伴一起尋找到了一種更有選擇性、更安全的治療方法。他們將可修改DNA的化合物苯醌與核酸共同構建了一種可結合到特定基因序列的結合物,從而達到靶向治療的目的。重要的是,這些化學修飾是可逆的,從而盡量減少苯醌-核酸結合物對非靶點的基因的傷害。這項研究可能為我們研發低劑量、低毒性和靶向性化療打開新的大門。
Drug development: More carefulchemotherapy
Chemical agents that bind and reversibly modifyspecific DNA sequences could provide the foundation for safer anticancertreatments. Seeing that many current chemotherapy agents kill cancer cells byintroducing irreversible modifications at any site in the genome—damagingnon-target cells while also depleting the amount of drug available to fightcancer—Steven Rokita at the University of Maryland, along with colleagues inChina, sought a more selective and safer approach to treatment. They combinedDNA-modifying compounds known as quinone methides with nucleic acid constructsthat specifically bind particular gene sequences, allowing for targetedtreatment. Importantly, these chemical modifications are potentiallyreversible, minimizing the damage that occurs if the quinone methide-nucleicacid conjugates briefly bind the wrong DNA sequence. This could open doors forthe development of less toxic, targeted chemotherapies that are effective atlower doses
Abstract
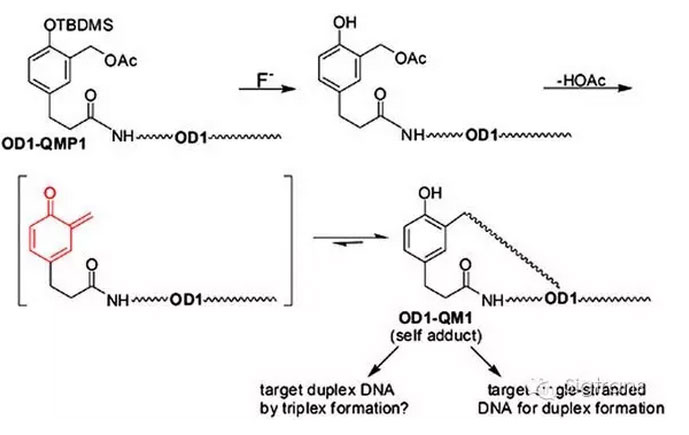
DNA alkylation and crosslinking remains a common andeffective strategy for anticancer chemotherapy despite its infamous lack ofspecificity. Coupling a reactive group to a sequence-directing component hasthe potential to enhance target selectivity but may suffer from prematuredegradation or the need for an external signal for activation. Alternatively,quinone methide conjugates may be employed if they form covalent but reversibleadducts with their sequence directing component. The resulting self-adductstransfer their quinone methide to a chosen target without an external signaland avoid off-target reactions by alternative intramolecular self-trapping.Efficient transfer is shown to depend on the nature of the quinone methide andthe sequence-directing ligand in applications involving alkylation of duplexDNA through a triplex recognition motif. Success required an electron-richderivative that enhanced the stability of the transient quinone methide intermediateand a polypyrimidine strand of DNA to associate with its cognatepolypurine/polypyrimidine target. Related quinone methide conjugates withpeptide nucleic acids were capable of quinone methide transfer from theirinitial precursor but not from their corresponding self-adduct. The activepeptide nucleic acid derivatives were highly selective for their complementarytarget.
Chengyun Huang, Yang Liu & Steven E Rokita. Targeting duplex DNA with the reversible reactivity of quinone methides. Signal transduction and targeted therapy, 1. (2016). doi:10.1038/sigtrans.2016.9